Balbharti Yuvakbharati English 11th Digest Chapter 1.1 Being Neighborly Notes, Textbook Exercise Important Questions and Answers.
Maharashtra State Board Class 11 English Yuvakbharati Solutions Chapter 1.1 Being Neighborly
11th English Digest Chapter 1.1 Being Neighborly Textbook Questions and Answers
Read the following statements and mark those that apply to you.
Question 1.
(i) I make friends easily.
(ii) I wish to be friends with someone but my friendship is rejected.
(iii) Someone has extended a hand of friendship towards me and I have not accepted it.
(iv) I have a large group of friends but no best buddy.
(v) I have a small group of close friends and have no wish to interact with anyone else.
(vi) I have cordial relationships with all but I cannot connect with anyone.
Answer:
(i) I make friends easily.
(iv) I have a large group of friends but no best buddy.

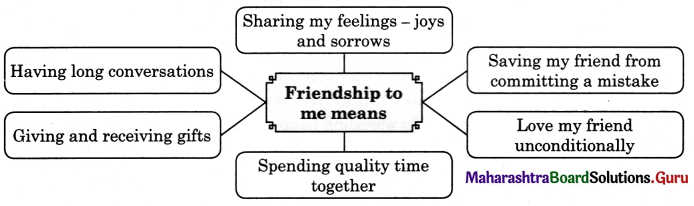
Complete the following web diagram.
Question (i)
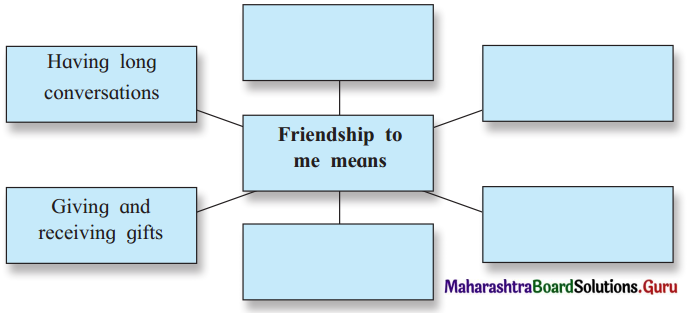
Answer:
Question (ii)
If you see someone lonely or sad you will –
(a) try to cheer the person by talking something pleasant.
(b) try to distract the person’s attention by doing some activity together.
(c) discuss the problem if the person wants to, give a patient hearing and also try to suggest some possible solutions.
(A1)
Question (i)
Jo’s decision to make friends with the lonely boy next door proves to be a good one. Elaborate. You may begin with ‘Jo was a bold, friendly and warm person…”
Answer:
Jo was bold, friendly and warm person who observed the boy next door closely and came to the conclusion that he was hungry for friends and fun. She was sad for the boy and felt it her neighborly duty to help the boy come out of his loneliness in her own way. She took a quick decision to catch the boys attention by throwing snowballs towards the window from where he was peeping.
She took the initiative to start an immediate conversation which was well-received by the boy. He invited her home and Jo readily accepted. Jo’s friendliness made the boy feel comfortable and he enjoyed Jo’s way of talking, her humour and most importantly, her companionship. He showed her his library and together they had a gala time which he never had before.

Question (ii)
Complete the following statements. (Answers are given directly in bold)
Answer:
- To Jo the fine house seemed like an enchanted house.
- Jo swept a path around the garden for Beth to walk in when the sun came out.
- Jo entered the old stone house carrying her broom.
- In order to tidy the room, Jo had whisked things into place.
Question (iii)
Bring out the contrast between the two houses with the help of the following points:
Answer:
| House of March | House of Laurence |
| 1. Old, brown house 2. Rather bare and shabby 3. Children played all around 4. A lively household having four girls and a loving mother | (a) Stately stone mansion (b) Stately stone mansion (c) Well kept grounds (d) All quiet, curtain down at the lower windows
No motherly face smiled at the windows |
(A2)
Question 1.
The traits of the characters you meet in the extract are jumbled. Sort them out and write them in the appropriate columns.
(Shy, bold, gruff, friendly, withdrawn, perceptive, empathetic, playful, lonely, happy, gentlemanly, frank, mature, dull, sharp, adventurous.)
| Jo | Laurie | Grandpa |
| adventurous | dull | gruff |
| bold | lonely | withdrawn |
| playful | gentlemanly | frank |
| happy | shy | sharp |
| friendly | mature | |
| empathetic | sharp | |
| perceptive | friendly | |

(A3)
Question (i)
Write down in your own words the way Laurie confirmed the names of the March sisters.
Answer:
The March family sometimes forgot to put the curtain down at the window and that helped Laurie, their neighbor, to observe minutely inside the March household. The sisters often call one another and lonely Laurie enjoys watching them having good time. That’s how he came to know that Beth is the one who is generally a home-bird but whenever she goes out, she carries a basket with her. Amy’s curly hair and Meg’s pretty face has also caught his attention.
Question (ii)
Give a brief account of the interaction between Grandpa and Jo.
Answer:
Grandpa and Jo had an interesting interaction as Jo had come out of her initial fear after having a closer look at him. Grandpa had overheard Jo’s comments on his portrait and Jo did not even try to deny any one of them. This pleased Grandpa immensely and he remembered Jo’s grandfather who was similarly brave and honest.
Jo frankly told Grandpa about the problem Laurie was facing because of his loneliness. She showed her concern and expressed the March sisters’ eagerness to help Laurie. They started talking informally about Hemmel family, Jo’s mother and he also invited Jo to join for tea which Jo courteously accepted. This interaction made Jo very satisfied as she could find out how good their neighbor was.
(A4)
Question (i)
Find proverbs, maxims and idioms related to ‘friendship’.
Answer:
- Birds of a feather flock together.
- A friend in need is a friend in deed.
- Friendship is love with understanding.
- To get on like a house on fire (idiom).
- Like two peas in a pod (idiom).
Question (ii)
The Extract deals with the atmosphere of two homes. Collect the words associated with –
- Home
- Library
- Garden
Answer:
- Home: old, brown, bare, shabby, stately stone mansion, comfort, luxury, big coach house, lovely things, rich curtains, lifeless, lawn, enchanted, hidden glories, full of splendour.
- Library: books, pictures, statues, little cabinets, coins, sleepy hollow chairs, queer tables, quaint tiles, open fireplace, bronzes.
- Garden: large, low hedge, vines, flowers.

(A5)
Change into indirect speech.
Question (a)
“Do you like your school?” asked the boy. “Don’t go to school I’m a businessman – girl, I mean”, answered Jo.
Answer:
The boy wanted to know whether she (Jo) liked school to which Jo answered quite emphatically that she did not go to school. She further added that she was a businessman and jovially corrected the gender.
Question (b)
Jo flourished her broom as she called out… “How do you do? Are you sick?
Laurie opened the window and croaked out as hoarsely as a raven…
“Better, thank you. I’ve had a bad cold, and been shut up a week.”
Answer:
Flourishing her broom Jo asked Laufie about his well-being and enquired whether he was sick. Laurie opened the window and croaked out as hoarsely as a raven thanking Jo for her concern and informed her that he was feeling better. He further added that he had been shut up a week as he had a bad cold.
Question (c)
“The pretty one is Meg, and the curly-haired one is Amy, I believe?” – Laurie.
“How did you find that out?” – Jo.
Answer:
Laurie wanted to confirm from Jo whether the pretty one was Meg and the curly-haired was Amy. With surprise in her voice Jo enquired how he(Laurie) had found that out.
Question (d)
“I’m not afraid of anything”, returned Jo with a toss of the head.
“I don’t believe you are !” exclaimed the boy.
Answer:
With a toss of the head Jo emphatically told that she was not afraid of anything. The boy was not surprised at her claim and agreed with her completely.
(A6)
Question (i)
Narrate in 100 words an incident, that illustrates the way a friend of yours ‘made you feel happy and accepted’, at some point in your life.
Answer:
That was my first day at school. I was just five years old. When my parents left me in school and I entered the classroom. I felt so lonely that I was about to cry. I was looking around, desperately trying to find out a known or a friendly face to talk to. Suddenly, there was a pat on my back and I saw a girl standing behind me.
She held my hand and took me to the bench where she was sitting. We became friends instantly. Till today we are the best friends. I shall be very grateful to her for her acceptance of me on the very first day at school.

Question (ii)
Give reasons, for us being reluctant to make friends with some strangers, but being comfortable with some, even after meeting them for the first time.
Answer:
Strangers are always mysteries for us. But some people have the inherent simplicity which instantly attract us towards them and we long to be friends with them. We feel comfortable in their company. But there is another category of people who have the attention-catching technique of blowing their own trumpets. It is difficult to carry on normal conversation with them as they are obsessed with their ownselves.
Question (iii)
Are friends different from neighbors? Are you friends with your neighbors? Give examples and write.
Answer:
It is not necessary to have one’s friend as one’s neighbor always. If it happens that way, then one is lucky. Friendship does not depend on the residence of a person and it can be different from the friendly relation one can have with one’s neighbor.
I am very fortunate to have a very friendly family as our neighbor. We are always there for one another at the hour of need as well as sharing happiness. For example, the owner of the apartment is a doctor and he is helping us with useful advices whenever anyone in our family falls sick. My mother is a teacher and she guides the children of our neighbor with their difficulties in studies. A good neighbor is always an asset.
Question (iv)
Make a note about how people amused themselves in earlier times without TV, internet or social media for entertainment.
Answer:
In earlier times when TV, internet and social media did not make people slaves of these sorts of entertainments, people used to socialize a lot. They used to meet their friends and relatives, talk to them over a telephone, make enquirers about each other’s well-beings and exchange ideas. The human connections were more and people used to share their joys and sorrows. Gone are those days of personal relationships which have been taken over by the modern technology enslaving people.

(A7)
Question 1.
Use your imagination and extend the story in about 100 to 130 words.
Answer:
Jo had a nice time with Laurie and his grandpa having tea and snacks which she enjoyed thoroughly. Both of them were very interesting characters, nice to talk to and Jo had an entertaining evening. She was excited to be acquainted with a friendly neighbor which she had always longed for. She was happy to go back home with so much of positive feelings about their neighbor who had been a mystery for her and her family.
Her entire family always felt sad for the lonely boy Laurie but nobody could approach him for helping him. She was extremely delighted to know Laurie and his grandpa who were courteous enough to invite her for tea. She was in a hurry to share her excitement with her family. “Ah! what a pleasant day it was !” she whispered.
(A8) Project:
If you are social, like to meet new people, can emphatise and connect with peole easily, make a list of careers available to you and write in brief about them. For example: Human Resource Development or HRD.
Yuvakbharati English 11th Digest Chapter 1.1 Being Neighborly Additional Important Questions and Answers
Question 1.
Jo doesn’t want to be a pussy-cat because –
Answer:
Pussy cat symbolises lethargy. Jo was always on the look out for excitements and thrills. She was an adventurous girl who does not want to idle away her time sleeping like a pussy-cat and enjoy the warmth of the fireplace on a cold winter afternoon. She would rather find out something interesting to spend her time.
Question 2.
Guess the meaning of‘hidden glories’ in the context of the mansion mentioned in the story.
Answer:
The expression has been used in the context of the mansion where Laurie lives. It has glimpses of lovely things and a look of an enchanted house, which probably hides lots of attractions inside.

Question 3.
Explain: “That boy is suffering for society and fun”.
Answer:
The young boy Laurie is lonely and longs for having fun with friends of his age-group, play with them and enjoy life the way a boy of his age does. The absence of company of friends and fun has made him dull which is affecting him like a disease.
Question 4.
Discuss ‘as dull as tombs’ and name the figure of speech.
Answer:
The figure of speech is ‘Simile’ as the dullness of the house is directly compared to the serious and dull atmosphere in a tomb. The boy meant by the expression that his house is very boring.
Question 5.
Complete the sentence: ‘a little gentleman’ means.
Answer:
The young boy is referred to as ‘a little gentleman’ here as he talks and behaves decently with others. He has a good upbringing which has taught him to welcome guests at his place by presenting himself as well as his room tidily.
Question 6.
Make a list of gifts you give/receive to/from your friends.
The gifts I usually give/receive to/from my friends are:
- books
- cosmetics
- various food items
- accessories

Question 7.
Complete the sentence in your own words : Hunger is related to food. Laurie is ‘hungry’ for –
Answer:
Laurie is a lonely young boy who is hungry for spending happy times both at home and with friends. He belongs to a rich family where he gets everything but suitable companions to have fun with. That is why he longs for food for his mind, that is, happy times with friends.
Question 8.
Laurie has
(i) _________
(ii) _________
(iii) _________
He doesn’t have
(i) __________
(ii) _________
(iii) _________
Answer:
Laurie
(i) a rich house filled with loneliness,
(ii) a kind but indifferent grandpa,
(iii) half a dozen servants and a tutor Mr. Brooke,
He doesn’t have
(i) his mother.
(ii) friends and companions,
(iii) any one to go out with.
Question 9.
Describe the effect of Laurie’s words on Jo.
Answer:
Jo started talking with Laurie frankly. Her words had lots of positive effects on Laurie as he was longing for exactly those things which Jo mentioned. For their first meeting, Jo was a bit blunt but Laurie liked her bluntness since he could recognize Jo’s sincerity and kindness hidden in those words. He started feeling comfortable in Jo’s company and enjoyed every bit of humorous description of Aunt March.
Question 10.
Find out what ‘good breeding’ means.
Answer:
‘Good breeding’ means that a person is well-behaved, polite, cultured and refined, which are the results of his upbringing, training as well as family atmosphere.

Question 11.
List some of the things that you need in order to be happy.
Answer:
Things that I need in order to be happy are –
(i) a supportive family and dependable friends.
(ii) hobbies to occupy myself during my free time.
(iii) a healthy life for me as well as my family members.
(iv) sufficient money earned from a satisfying career.
Question 12.
“A fellow can’t live on books” – Explain.
Answer:
A fellow, of course, cannot live on books though books are his emotional suppdrt and in many ways, his best friend. But he also needs someone, a companion, with whom he can share his feelings, fulfill his curiosities, have fun, etc. Human contact is a necessity in a person’s life since a few words, an exchange of ideas collected from the books can work wonders giving immense pleasure.
Question 13.
List the things that Jo notices in the portrait.
Answer:
- The gentleman in the portrait is not as handsome as her own grandfather.
- Though the gentleman is having a grim face, his kind eyes assure that there is nothing to be afraid of him.
- From his looks, it appears that he has tremendous will-power.
Question 14.
Find out the reason for Jo’s dismay.
Answer:
Jo loudly expressed her opinions on Laurie’s grandfather, while looking at his portrait. When she came to know that the gentleman had heard all her comments, she felt embarrassed. She felt uncomfortable to face the old gentleman and felt like running away.
Question 15.
Complete the sentence.
Answer:
In spite of Jo’s apprehensions, Grandpa is –
- having kinder eyes than what the painting shows.
- having a shy twinkle in his eyes which could lessen Jo’s fear.
- quite a friendly gentleman.

Question 16.
Discuss what Jo meant by –
(i) “only trying to be neighborly, Sir.”
(ii) seems a little lonely.
(iii) splendid Christmas present.
Answer:
(i) By saying, only trying to be neighborly, Sir”. Jo means she just wanted to be friendly with Laurie as he was her neighbor. She strongly felt that neighbors should know each other well.
(ii) Jo had observed Laurie now and again and she felt Laurie badly needed company since he always eagerly looked at his neighbors as if he was missing the fun they were having. To her, he appeared to be a lonely boy longing for enjoyment with friends.
(iii) Jo remembered the beautiful Christmas present that was sent to the March family by their neighbor Mr. Laurence and she felt it was a nice gesture by their neighbor.
Question 17.
Guess the meaning of the phrase “go on being neighborly” in the context.
Answer:
The phrase “go on being neighborly” in the context of the story means being friendly and helpful to the people living in one’s neighborhood.
Question 18.
Bring out the contrast in the lives of Jo and Laurie in a few lines.
Answer:
Jo belonged to a happy family who according to Laurie, had always good times together. Laurie was hungry to have company of friends and was a lonely boy. Jo had a loving mother who used to take care of her children but, Laurie was a motherless child who badly missed his mother. Jo was frank and innocent as any child of her age, whereas, Laurie’s loneliness was sickening for him.

Question 19.
I’m happy as a cricket here. (Name and explain the figure of speech)
Answer:
Simile. The happiness of Jo is directly compared to the happiness of the insect cricket.
Question 20.
Guess the meaning of the word ‘affair’ in the context.
Answer:
The word ‘affair’ in the context means ‘responsibility/matter’.
Comprehension:
Read the extract and complete the activities given below.
Global Understanding:
Question 1.
Complete the table.
The traits of Jo and Laurie are jumbled. Sort them out and write them in appropriate columns.
(companionless, adventurous, empathetic, unenergetic) (Answers are given directly)
Answer:
| Jo | Laurie |
| adventurous | companionless |
| empathetic | unenergetic |
Question 2.
Pick up the statements which confirm the theme of this passage.
(a) This passage is about Jo’s family not putting down the curtain.
(b) This passage is about Jo’s confirmation about Laurie’s loneliness.
(c) This passage is about Laurie’s habit of peeping at Jo’s family.
(d) This passage brings out the contrast in the lives of Jo and Laurie.
Answer:
(b) This passage is about Jo’s confirmation about Laurie’s loneliness.
(d) This passage brings out the contrast in the lives of Jo and Laurie.
Question 3.
Complete the sentences in column ‘A’ by matching them with the clues in column ‘B’
| Column ‘A’ | Column ‘B’ |
| 1. Laurie inspite of being inquisitive asked no questions as ____________ . | (a) Laurie seldom laughed aloud |
| 2. Maid was surprised as ____________ . | (b) That indicated his good breeding |
| 3. Jo was elated ____________ . | (c) As she was successful in making Laurie laugh |
| 4. Jo found happiness in ____________ . | (d) Reading books |
Answer:
| Column ‘A’ | Column ‘B’ |
| 1. Laurie inspite of being inquisitive asked no questions as ____________ . | (a) That indicated his good breeding |
| 2. Maid was surprised as ____________ . | (b) Laurie seldom laughed aloud |
| 3. Jo was elated ____________ . | (c) Reading books |
| 4. Jo found happiness in ____________ . | (d) As she was successful in making Laurie laugh |

Question 4.
Complete the following statement with four correct information from the extract.
Jo felt Laurie needs cheering up because:
- ________
- ________
- ________
- ________
Answer:
- He seemed lonely
- she was being neighborly
- She was social and empathetic
- Laurie looked expectantly at the sisters as they bad good time.
Complex Factual:
Question 1.
What were Jo’s queries to Laurie when they had talked for the first time?
Answer:
Jo wanted to know whether Laurie was sick, how he amused himself, his liking for books and if he had any visitor or not.
Question 2.
Mention any two outcomes of Jo’s visit to Laurie’s place.
Answer:
Jo’s visit made Laurie excited in the expectation of getting a companion which he never had. It also helped him to come out of his shyness and converse with Jo freely.
Question 3.
What was Jo’s suggestion to do away with Laurie’s loneliness.
Answer:
Jo assured Laurie that the curtain at her place would never be drawn so that Laurie can spend time looking at their activities. She also suggested that Laurie could come to their home and get himself entertained by. all the members of her family.

Question 4.
Why was Laurie’s grandfather impressed with Jo?
Answer:
Laurie’s grandfather was impressed by Jo’s spirited answers like her grandfather. He also appreciated that she was brave and honest as her grandfather was.
Question 5.
Mention any four changes that occurred in grandfather after meeting Jo.
Answer:
- Grandfather shed his strict countenance.
- He invited Jo to come over for tea.
- He promised to come over to meet Jo’s mother.
- He offered Jo his arm with old fashioned courtesy (indicating his gratitude for her neighborly arrival)
Inference / Interpretation / Analysis:
Question 1.
Complete the following statement.
Jo wanted to help Laurie because –
Answer:
Jo wanted to help Laurie because Laurie was unwell and he felt lonely as he had no one of his age at home. He deserved to have fun.
OR
Give reasons
“The big eyes brightened and the mouth began to smile”.
Answer:
The brightness in the eyes of Laurie and his smiling face are proofs of his happiness of having a possible friendship with Jo. When Jo threw snowballs at Laurie, he could feel Jo’s eagerness to talk to him. His loneliness has always made him unhappy and this gesture of Jo is a welcome change for him.
Question 2.
Complete the following sentence Mr. Laurie was a Tittle gentleman’ as …
Answer:
Mr. Laurie was a Tittle gentlemen’ because he was known for offering due respect to the guest who was coming to his place. He prepared himself decently by brushing his pate, pulling on a fresh set of clothing and making an attempt to clean his room. He followed the same routine for Jo’s arrival to his house also.

Question 3.
Point out the reason for Laurie’s minute observation of Jo’s family.
Answer:
Laurie spent his lonely time looking at the fun Jo’s family was having. He enjoyed watching each member of the family eagerly as he missed all those good times at his own home. He did not have his mother and he loved these girls in the company of their mother.
Question 4.
Mention the impact of Jo’s narratives on Laurie –
1. …………….
2. ……………..
Answer:
1. Laurie enjoyed her narrative immensely and he laughed out aloud.
2. He found a sudden merriment in otherwise dull mood owing to his illness.
Question 5.
Jo wasn’t scared of Laurie’s grandfather. Give evidence from the passage quoted to you.
Answer:
Jo was a bold girl who spoke her mind when it was needed. As she looked at Mr. Laurence’s portrait she found his eyes to be kind and grew fond of him instantly. She found him to be compassionate as she spoke to him and was confident that there was nothing to be scared of that gentleman.
Question 6.
Complete the boxes with information.
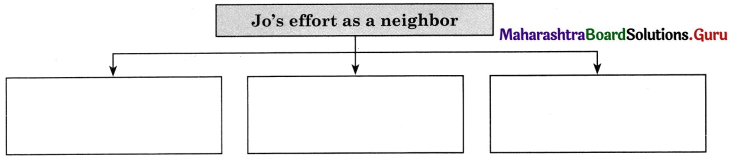
Answer:

Personal Response:
Question 1.
“Girls are quiet and like to play nurse”. Do you Agree or Disagree? Justify your answer.
Answer:
I do not agree to the statement. There is no hard and fast rule about this impression about girls. There may be many boys who are very quiet and also good at nursing. It depends on the nature of a person whether he/ she is quiet, or noisy or talkative. The ability to nurse somebody is also dependant on the ability of a person irrespective of any gender.

Question 2.
Enlist the gifts that you receive from or give to your friends,
Answer:
- Books
- Wind Chimes
- Coffee mugs
- Photo frames
Question 3.
Give your suggestions in two sentences. How you will cheer up one of your lonely classmates.
Answer:
I can cheer up my lonely classmate by giving him/her company and involving in some activities together. We can sit together in the classroom, share our tiffins and invite him/her at my place on holidays.
Question 4.
What are you afraid of? Explain your answer.
Answer:
Generally I am not afraid of anything and a carefree type of person. But sometimes I worry about the loss of my near and dear ones as I am very attached to my family and friends.
Question 5.
What do you fear the most? why?
Answer:
As a student I fear examination especially the public exams as they decide the future course of action. There is always an element of uncertainty which brings in fear for exams among students.
Question 6.
How do you help your neighbor?
Answer:
I help my neighbor by making myself available when they need me. I also extend courtesy calls when I meet them.
Language Study:
Question (i)
The idea amused Jo who liked to do daring things
Answer:
Jo liked to do daring things and the idea amused her.
Question (ii)
The boy is suffering for society.
Answer:
The boy has been suffering for society.

Question (iii)
What a cozy room this is! (Rewrite as a statement)
Answer:
This room is indeed very cozy.
Question (iv)
Laurie forgot his bashfulness and grew sociable. (Remove ‘and’ to make it a simple sentence)
Answer:
Forgetting his bashfulness Laurie grew sociable.
Question (v)
Her face was very friendly and her sharp voice unusually gentle.
(Use ‘not only but also’ and rewrite)
Answer:
Her face was not only very friendly but her sharp voice was unusually gentle also.
Question (vi)
She had been so simply taught that there was no nonsense in her head. (Use ‘too’)
Answer:
She had been too simply taught to have any nonsense in her head.
Question (vii)
Laurie enjoyed that immensely. (Use ‘enjoyment’and rewrite)
Answer:
Laurie’s enjoyment at that was immense.
Question (vii)
Jo liked his good breeding. (Frame a Wh-question to get the underlined part as an answer)
Answer:
What did Jo like in him?
Question (viii)
For a minute a wild desire to run away possessed her. (Change the voice)
Answer:
For a minute she was possessed by a wild desire to run away.
Question (ix)
He isn’t as handsome as my grandfather, but I like him. (Use ‘Though’)
Answer:
Though he isn’t as handsome as my grandfather, I like him.

Question (x)
He seems a little lonely. (Frame a question to get the underlined part as answer)
Answer:
How does he seem?
Question (xi)
I shall come and see your mother. (Use a modal auxiliary showing ‘obligation’)
Answer:
I must come and see your mother.
Vocabulary:
Question 1.
Match the words in Column ‘A’ with their meanings in Column ‘B’.
Answer:
| Column ‘A’ | Column ‘B’ |
| 1. queer | (a) bold |
| 2. dismal | (b) frail |
| 3. daring | (c) unusual |
| 4. weak | (d) dull |
Answer:
| Column ‘A’ | Column ‘B’ |
| 1. queer | (c) unusual |
| 2. dismal | (d) dull |
| 3. daring | (a) bold |
| 4. weak | (b) frail |
Question 2.
Mention any 4 adjectives that describe the traits of Jo’s character in the extract.
Answer:
Character
Jo
Adjectives
- Cheerful
- Empathetic
- Sociable
- Kind

Question 3.
Give antonyms of the following words,
- rude
- splendid
- funny
- frank
Answer:
- polite
- ordinary
- serious
Question 4.
Give antonyms of the following using prefix.
Answer:
1. interesting × uninteresting
2. afraid × unafraid
Question 5.
Give one word for:
Answer:
- of a voice: low and rough – Gruff
- Lacking courage – Cowardly
- unpleasant – Dreadful
- very large or great – Tremendous
Glossary:
- shovel – spade
- mischievous – naughty
- to doze – to sleep lightly
- groves – group of trees
- hedge – row of bushes
- shabby – broken down/dilapidated
- stately – grand
- mansion – big house
- betokening – a sign of something
- glimpses – brief/faint looks
- frolicked – played fun games
- enchanted – attractive/ fascinating
- splendors – richness / luxury
- behold – look/see
- scandalizing – shocking /disgusting
- queer – odd/unusual/funny
- dismal – dull row – noise
- flutter – tremendous/full of
- pate – head
- parlor – sitting room
- briskly – quickly
- comforting – soothing
- sociable – friendly
- cozy – comfortable
- hearth – floor of fireplace
- whisked – removed
- beckoned – called
- twitching – shivering
- splendid – grand/superb
- bother- trouble/nuisance
- acquainted – be familiar
- blunt – frank/straightforward
- fidgety – restless
- poodle – a bread of dog
- immensely – vastly/very much
- tweaked – pulled
- elated – delighted
- trifle – little
- quaint – old-fashioned /unusual/attractive
- velour – woven fabric
- grim – ill-tempered/stern
- gruff – rough
- cowardly – fearful
- twinkle – shining
- dreadful – terrible
- courtesy – politeness
- colored up – embarrassed
- to wait on – act as an attendant to
- pranced – walked in an energetic way
- wicked – playfully mischievous
- affair – matter/responsibility
- good breeding – being raised well/ the result of good upbringing and training for good manners.