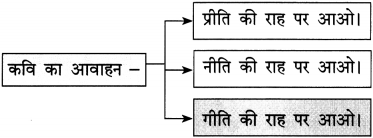
Balbharti Maharashtra State Board Hindi Yuvakbharati 12th Digest Chapter 3 सच हम नहीं; सच तुम नहीं Notes, Textbook Exercise Important Questions and Answers.
Maharashtra State Board 12th Hindi Yuvakbharati Solutions Chapter 3 सच हम नहीं; सच तुम नहीं
12th Hindi Guide Chapter 3 सच हम नहीं; सच तुम नहीं Textbook Questions and Answers
कृति-स्वाध्याय एवं उत्तर
आकलन
प्रश्न 1.
(अ) कविता की पंक्ति पूर्ण कीजिए :
(1) अपने हृदय का सत्य, – ……………………….
(2) आदर्श हो सकती नहीं, – ……………………….
(3) बेकार है मुस्कान से ढकना, – ……………………….
(4) अपने नयन का नीर, – ……………………….
उत्तर :
(1) अपने हृदय का सत्य, अपने-आप हमको खोजना।
(2) आदर्श हो सकती नहीं, तन और मन की भिन्नता।
(3) बेकार है मुस्कान से ढकना, हृदय की खिन्नता।
(4) अपने नयन का नीर, अपने-आप हमको पोंछना।
![]()
(आ) लिखिए :
(a) जीवन यही है
(1) जीवन यही है –
(i) …………………………….
(ii) …………………………….
(iii) …………………………….
(iv) …………………………….
उत्तर :
जीवन यही है –
(i) नत न होना।
(ii) पंथ भूलने पर भी न रुकना।
(iii) हार देखकर भी न झुकना।
(iv) मृत्यु को भी जीत लेना।
(b) मिलना वही है –
(1) मिलना वही है – ……………………….
(2) यह जिंदगी जिंदगी नहीं है – ……………………….
(3) हर राही को इससे दिशा मिलती है – ……………………….
(4) कवि तब तक इस राह को सही नहीं मानेगा – ……………………….
उत्तर :
(1) जो मँझधार को मोड़ दे।
(2) जो सिर्फ पानी-सी बहती रहे।
(3) भटकने के बाद।
(4) जब तक जीवन बँधा होगा और जब तक प्यार पर दुख की गहरी छाया होगी।
शब्द संपदा
प्रश्न 2.
प्रत्येक शब्द के दो पर्यायवाची शब्द लिखिए :
(1) पंथ – [ ] [ ]
(2) काँटा – [ ] [ ]
(3) कुसुम – [ ] [ ]
(4) हार – [ ] [ ]
उत्तर :
(1) पंथ – [ रास्ता ] [ डगर ]
(2) काँटा – [ शूल ] [ कंटक ]
(3) कुसुम – [ पुष्प ] [ प्रसून ]
(4) हार – [ पराजय ] [ पराभव ]
अभिव्यक्ति
प्रश्न 3.
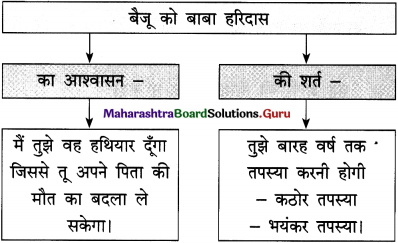
(अ) ‘जीवन निरंतर चलते रहने का नाम है’, इस विचार की सार्थकता स्पष्ट कीजिए।
उत्तर :
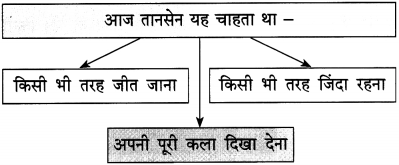
जीवन का उद्देश्य निरंतर आगे-ही-आगे बढ़ते रहना है। जीवन में ठहराव आने को मृत्यु की संज्ञा दी जाती है। अनेक महापुरुषों ने अपने उद्देश्य की पूर्ति के लिए जीवनभर संघर्ष किया है और उनका नाम अमर हो गया है। जीवन का मार्ग आसान नहीं ३ है। उस पर पग-पग पर कठिनाइयाँ आती रहती हैं। इन कठिनाइयों 1 से उसे जूझना पड़ता है। उसमें हार भी होती है और जीत भी होती ३ है। असफलताओं से मनुष्य को घबराना नहीं चाहिए।
![]()
बल्कि उनका ३ दृढ़तापूर्वक सामना करके उसमें से अपना मार्ग प्रशस्त करना और ३ निरंतर आगे बढ़ते रहना चाहिए। एक दिन मंजिल अवश्य मिलेगी। जीवन संघर्ष कभी न खत्म होने वाला संग्राम है। इसका सामना करने का एकमात्र मार्ग है निरंतर चलते रहना और हर स्थिति में संघर्ष जारी रखना।
(आ) ‘संघर्ष करने वाला ही जीवन का लक्ष्य प्राप्त करता है, इस विषय पर अपने विचार प्रकट कीजिए।
उत्तर :
दुनिया में दो प्रकार के मनुष्य होते हैं। एक वे जो सामान्य रूप से चलनेवाली जिंदगी जीना पसंद करते हैं और आगे बढ़ने के लिए किए जानेवाले उठा-पटक को पसंद नहीं करते। दूसरे तरह के वे लोग होते हैं, जो अपना लक्ष्य निर्धारित कर लेते हैं और उसे प्राप्त करने के लिए संघर्ष का रास्ता चुनते हैं। ऐसे लोगों का जीवन आसान नहीं होता। इन्हें पग-पग पर विभिन्न रुकावटों का सामना करना पड़ता है।
पर ऐसे लोग इन रुकावटों से डरते नहीं, बल्कि हँसते-हँसते इनका सामना करते हैं। सामना करने में अनेक बार असफलता भी इनके हाथ लगती है। पर ये इससे हताश नहीं होते। ये फिर अपनी गलतियों को सुधारते हैं और नए सिरे से संघर्ष करने में जुट जाते हैं। परिस्थितियाँ कैसी भी हों, वे न झुकते हैं और न हताश होते हैं। उनके सामने सदा उनका लक्ष्य होता है। उसे प्राप्त करने के लिए वे निरंतर संघर्ष करते रहते हैं।
ऐसे लोग अपनी निष्ठा और लगन के बल पर एक-न-एक दिन अवश्य सफल हो जाते हैं। वे संघर्ष के बल पर अपने जीवन का लक्ष्य प्राप्त करके रहते हैं।
रसास्वादन
प्रश्न 4.
‘आँसुओं को पोंछकर अपनी क्षमताओं को पहचानना ही जीवन है’, इस सच्चाई को समझाते हुए कविता का रसास्वादन कीजिए।
उत्तर :
डॉ. जगदीश गुप्त द्वारा लिखित कविता ‘सच हम नहीं, सच तुम नहीं’ में जीवन में निरंतर संघर्ष करते रहने का आह्वान किया गया है।
कवि पानी-सी बहने वाली सीधी-सादी जिंदगी का विरोध करते हुए संघर्षपूर्ण जीवन जीने की बात करते हैं। वे कहते हैं, जो जहाँ भी हो, उसे संघर्ष करते रहना चाहिए।
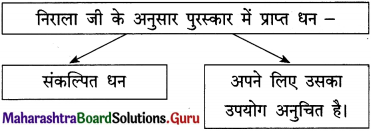
संघर्ष में मिली असफलता से निराश होने की आवश्यकता नहीं है। ऐसी हालत में हमें किसी के सहयोग की आशा नहीं करनी। हमें अपने आप में खुद हिम्मत लानी होगी और अपनी क्षमता को पहचानकर नए सिरे से संघर्ष करना होगा। मन में यह विश्वास रखकर काम करना होगा कि हर राही को भटकने के बाद दिशा मिलती ही है और उसका प्रयास व्यर्थ नहीं जाएगा। उसे भी दिशा मिलकर रहेगी।
कवि ने सीधे-सादे शब्दों में प्रभावशाली ढंग से अपनी बात कही है। अपनी बात कहने के लिए उन्होंने ‘अपने नयन का नीर पोंछने’ शब्द समूह के द्वारा हताशा से अपने आपको उबार कर स्वयं में नई शक्ति पैदा करने तथा ‘आकाश सुख देगा नहीं, धरती पसीजी है नहीं’ से यह कहने का प्रयास किया है कि भगवान तुम्हारी सहायता के लिए नहीं आने वाले हैं और धरती के लोग तुम्हारे दुख से द्रवित नहीं होने वाले हैं। इसलिए तुम स्वयं अपने आप को सांत्वना दो और नए जोश के साथ आगे बढ़ो। तुम अपने लक्ष्य पर पहुँचने में अवश्य कामयाब होंगे।
साहित्य संबंधी सामान्य ज्ञान
प्रश्न 5.
जानकारी दीजिए :
(अ) ‘नई कविता’ के अन्य कवियों के नाम –
(आ) कवि डॉक्टर जगदीश गुप्त की प्रमुख साहित्यिक कृतियों के नाम –
उत्तर :
(अ) ‘नई कविता’ के अन्य कवियों के नाम – [रामस्वरूप चतुर्वेदी, विजयदेव साही]
(आ) कवि डॉक्टर जगदीश गुप्त की प्रमुख साहित्यिक कृतियों के नाम – [‘नाँव के पाँव, शब्द दंश, हिम विद्ध, गोपा-गौतम’] (काव्य संग्रह), ‘शंबूक’ (खंडकाव्य), ‘भारतीय कला के पदचिहन, नयी कविता : स्वरूप और समस्याएँ, केशवदास’ (आलोचनाएँ) तथा ‘नयी कविता’ (पत्रिका)।]
प्रश्न 6.
निम्नलिखित वाक्यों में अधोरेखांकित शब्दों का लिंग परिवर्तन कर वाक्य फिर से लिखिए :
(1) बहुत चेष्टा करने पर भी हरिण न आया।
उत्तर :
बहुत चेष्टा करने पर भी हरिणी न आई।
(2) सिद्धहस्त लेखिका बनना ही उनका एकमात्र सपना था।
उत्तर :
सिद्धहस्त लेखक बनना ही उनका एकमात्र सपना था।
![]()
(3) तुम एक समझदार लड़की हो।
उत्तर :
तुम एक समझदार लड़के हो।
(4) मैं पहली बार वृद्धाश्रम में मौसी से मिलने आया था।
उत्तर :
मैं पहली बार वृद्धाश्रम में मौसा से मिलने आया था।
(5) तुम्हारे जैसा पुत्र भगवान सब को दे।
उत्तर :
तुम्हारी जैसी पुत्री भगवान सब को दे।
(6) साधु की विद्वत्ता की धाक दूर-दूर तक फैल गई थी।
उत्तर :
साध्वी की विद्वत्ता की धाक दूर-दूर तक फैल गई थी।
(7) बूढ़े मर गए।
उत्तर :
बुढ़ियाँ मर गईं।
(8) वह एक दस वर्ष का बच्चा छोड़ा गया।
उत्तर :
वह एक दस वर्ष की बच्ची छोड़ी गई।
(9) तुम्हारा मौसेरा भाई माफी माँगने पहुंचा था।
उत्तर :
तुम्हारी मौसेरी बहन माफी माँगने पहुंची थी।
(10) एक अच्छी सहेली के नाते तुम उसकी पारिवारिक पृष्ठभूमि का अध्ययन करो।
उत्तर :
एक अच्छे मित्र के नाते तुम उसकी पारिवारिक पृष्ठभूमि का अध्ययन करो।
Hindi Yuvakbharati 12th Digest Chapter 3 सच हम नहीं; सच तुम नहीं Additional Important Questions and Answers
कृतिपत्रिका के प्रश्न 2 (अ) तथा प्रश्न 2 (आ) के लिए
पद्यांश क्र. 1
प्रश्न. निम्नलिखितपद्यांशपढ़कर दी गई सूचनाओं के अनुसार कृतियाँ कीजिए :
कृति 1 : (आकलन)
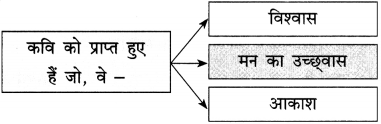
प्रश्न 1.
कृति पूर्ण कीजिए :
उत्तर :
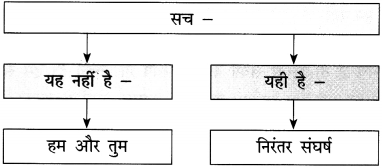
![]()
प्रश्न 2.
जीवन का संदेश
(i) …………………………….
(ii) …………………………….
(iii) …………………………….
(iv) …………………………….
उत्तर :
जीवन का संदेश –
(i) जीवन में कहीं जड़ता नहीं होनी चाहिए।
(ii) अपनी-अपनी जगह पर खुद से लड़ाई जारी रखनी चाहिए।
(iii) हर तरह की परिस्थिति का सामना करने की तैयारी होनी चाहिए।
(iv) इन्सान को कभी टूटना-हारना नहीं चाहिए।
प्रश्न 3.
उत्तर लिखिए :
(1) नत होने से मृत जैसा होने की तुलना की गई है इससे – …………………………….
(2) काँटे चुभे, कलियाँ खिलें का अर्थ – …………………………….
उत्तर :
(1) नत होने से मृत जैसा होने की तुलना की गई है इससे – [डंठल से झरे फूल से।]
(2) काँटे चुभे, कलियाँ खिलें का अर्थ – [स्थिति चाहे प्रतिकूल हो अथवा अनुकूल।]
पदयांश क्र. 2
प्रश्न. निम्नलिखितपद्यांशपढ़कर दी गई सूचनाओं के अनुसार कृतियाँ कीजिए :
कृति 1 : (आकलन)
प्रश्न 1.
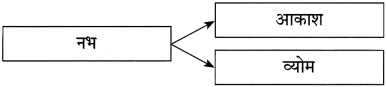
पद्यांश पर आधारित दो ऐसे प्रश्न तैयार कीजिए जिनके उत्तर निम्नलिखित शब्द हों :
(1) आकाश
(2) धरती
उत्तर :
(1) कौन सुख नहीं देगा?
(2) कौन नहीं पसीजती है?
कृति 2 : (शब्द संपदा)
प्रश्न 1.
प्रत्येक शब्द के दो-दो पर्यायवाची शब्द लिखिए :
(1) फूल – [ ] [ ]
(2) नीर – [ ] [ ]
(3) नयन – [ ] [ ]
(4) धरती – [ ] [ ]
उत्तर :
(1) फूल – [ पुष्प ] [ कुसुम ]
(2) नीर – [ अंबु ] [ जल ]
(3) नयन – [ चक्षु ] [ आँख ]
(4) धरती – [ पथ्वी ] [ अवनि ]
![]()
(2) निम्नलिखित शब्दों के विरुद्धार्थी शब्द लिखिए :
(1) तोड़ना x …………………………
(2) सच x …………………………
(3) दुख x …………………………
(4) बेकार x …………………………
उत्तर :
(1) तोड़ना x जोड़ना
(3) दुख x सुख
(2) सच x झूठ
(4) बेकार x साकार।
रसास्वादन मुद्दों के आधार पर
कृतिपत्रिका के प्रश्न 2 (इ) के लिए
प्रश्न 1.
निम्नलिखित मुद्दों के आधार पर सच हम नहीं; सच तुम नहीं’ कविता का रसास्वादन कीजिए।
उत्तर :
(1) रचना का शीर्षक : सच हम नहीं; सच तुम नहीं।
(2) रचनाकार : डॉ. जगदीश गुप्त।
(3) कविता की केंद्रीय कल्पना : प्रस्तुत कविता में निरंतर आगे बढ़ते रहने, संघर्ष करते रहने और मार्ग में आनेवाली रुकावटों की परवाह न करके अपने लक्ष्य की प्राप्ति की ओर अग्रसर होने की प्रेरणा दी गई है। यही इस कविता की केंद्रीय कल्पना है।
(4) रस-अलंकार : –
(5) प्रतीक विधान : इस कविता में संघर्ष का मार्ग त्याग कर नत हो जाने यानी किसी की अधीनता स्वीकार कर लेने वाले को मृतक के समान हो जाना कहा गया है। कवि ने इस तरह के मृत व्यक्ति के लिए ‘डाल से झड़े हुए फूल’ का प्रतीक के रूप में उपयोग किया है।
(6) कल्पना : जीवन में दृढ़तापूर्वक संघर्ष का मार्ग अपनाना और निराश हुए बिना उस पर अडिग रहना ही जीवन की एकमात्र सच्चाई है।
(7) पसंद की पंक्तियाँ तथा प्रभाव : कविता की पसंद की पंक्तियाँ इस प्रकार हैं :
अपने हृदय का सत्य,
अपने आप हम को खोजना।
अपने नयन का नीर,
अपने आप हम को पोंछना।.
इन पंक्तियों में अपनी समस्याओं को पहचानने और उनका समाधान ढूँढ़ने के लिए बिना किसी की सहायता की उम्मीद किए स्वयं कमर कस कर तैयार होने की प्रेरणा मिलती है।
(8) कविता पसंद आने का कारण : कवि ने इस पंक्ति में यह बताया है कि संघर्ष में असफलता हाथ लगे, तो भी निराश होने की जरूरत नहीं है। हमें अपने आप अपनी आँखों के आँसू पौंछकर फिर से हिम्मत के साथ संघर्ष में जुट जाना है।
व्याकरण
अलंकार :
प्रश्न 1.
निम्नलिखित काव्य पंक्तियों में प्रयुक्त अलंकार पहचान कर उनके नाम लिखिए :
(1) हरि पद कोमल कमल से।
(2) झूठे जानि न संग्रही मन मुँह निकसे बैन। यहि ते मानहुँ किए, बातनु को बिघि नैन।
(3) पायो जी मैंने राम रतन धन पायो।
(4) हनूमान की पूँछ में लग न पाई आग। लंका सिगरी जल गई, गए निशाचर भाग।
(5) एक म्यान में दो तलवारें कभी नहीं रह सकतीं। किसी और पर प्रेम पति का नारियाँ नहीं सह सकी।
उत्तर :
(1) उपमा अलंकार
(2) उत्प्रेक्षा अलंकार
(3) रूपक अलंकार
(4) अतिशयोक्ति अलंकार
(5) दृष्टांत अलंकार।
![]()
रस:
प्रश्न 1.
निम्नलिखित काव्य पंक्तियों में प्रयुक्त रस पहचान कर लिखिए :
(1) काहु न तखा सो चरित विसेरना। सो सरूप नृप कन्या देखा।
मर्कट वदन भयंकर देही। देखत हृदय क्रोध मा तेही।
जेहि दिसि बैठे नारद फूली। सो दिसि तेहि न विलोकी भूली।
पुनि-पुनि मुनि उकसहिं अकुलाहीं। देखि दसा हर गन मुसुकाहीं।
(2) जो हौं तव अनुशासन पावौं
तौ चंद्रमहिं निचोरि चैल ज्यों आनि सुधा सिर नावौं।
कै पाताल दलौं व्यालावलि अमृत कुंड महि लावौ।
भेदि भुवन, करि भानु बाहियें तुरत राहु दै ताबौ।।
विबुध बैद बरबस आनौं धरि, तौ प्रभु अनुग कहावौं।
पटकौं मीच नीच मूषक ज्यों सबहिं को पाप कहावौं।
(3) कहुँ सुलगत कोउ चिता, कहुँ कोउ जात लगाई।
एक लगाई जात, एक की राख बुझाई।
विविध रंग की उठति ज्वाल दुर्गन्धिति महकति,
कहु चरबी सी चटचटाति कहुँ दह-दह दहकति।
उत्तर :
(1) हास्य रस
(2) वीर रस
(3) वीभत्स रस।
मुहावरे :
प्रश्न 1.
निम्नलिखित मुहावरों का अर्थ लिखकर वाक्य में प्रयोग कीजिए :
(1) गुड़ गोबर करना।
अर्थ : बने काम को बिगाड़ देना।
वाक्य : चित्रकार का चित्र तैयार था तभी एक छोटे बच्चे ने आकर उस पर ऐसी कूँची फिराई की सारा गुड़ गोबर हो गया।
(2) जहर का चूंट पीना।
अर्थ : अपमान को चुपचाप सह लेना।
वाक्य : मुंशीजी अपने चपरासी से कभी-कभी जब पैर दबा देने की बात करते थे तब वह जहर का यूंट पीकर रह जाता था।
(3) तिल का ताड़ बनाना।
अर्थ : छोटी बात को बढ़ा-चढ़ा कर कहना।
वाक्य : रमेश की बात विश्वास करने लायक नहीं होती, उसकी तो तिल का ताड़ बनाने की आदत है।
(4) मुट्ठी गर्म करना।
अर्थ : रिश्वत देना।
वाक्य : गाँवों में छोटा-मोटा काम करवाने के लिए भी अधिकारियों की मुट्ठी गर्म करनी पड़ती है।
![]()
(5) पत्थर की लकीर।
अर्थ : पक्की बात।
वाक्य : गाँव के लोग वकील साहब की बात को पत्थर की लकीर मानते थे।
काल परिवर्तन :
प्रश्न 1.
सूचनाओं के अनुसार काल परिवर्तन करके वाक्य फिर से लिखिए :
(1) वह पंथ भूल कर नहीं रुकता है। (पूर्ण भूतकाल)
(2) वह हार देख कर नहीं झुका। (सामान्य भविष्यकाल)
(3) आकाश सुख नहीं देगा। (अपूर्ण वर्तमानकाल)
(4) धरती नहीं पसीजती है। (पूर्ण वर्तमानकाल)
(5) हर एक राही को भटक कर दिशा मिलती है। (अपूर्ण भूतकाल)
उत्तर :
(1) वह पंथ भूल कर नहीं रुका था।
(2) वह हार देख कर नहीं झुकेगा।
(3) आकाश सुख नहीं दे रहा है।
(4) धरती नहीं पसीजी है।
(5) हर एक राही को भटक कर दिशा मिल रही थी।
वाक्य शुद्धिकरण :
प्रश्न 1.
निम्नलिखित वाक्य शुद्ध करके लिखिए :
(1) वह उसका काम कर रहा हैं।
(2) उस बगीचे में अनेकों फूले खिले हैं।
(3) पुस्तक की ढेर देख मैं दंग रह गया।
(4) उसे मात्र केवल दो दिन की छुट्टी चाहिएँ।
(5) मैं तुमको धन्यवाद करता हूँ।
उत्तर :
(1) वह अपना काम कर रहा है।
(2) उस बगीचे में अनेक फूल खिले हैं।
(3) पुस्तकों का ढेर देख में दंग रह गया।
(4) उसे केवल दो दिन की छुट्टी चाहिए।
(5) मैं तुम्हें धन्यवाद देता हूँ।
सच हम नहीं; सच तुम नहीं Summary in Hindi
सच हम नहीं; सच तुम नहीं कवि का परिचय
सच हम नहीं; सच तुम नहीं कवि का नाम : डॉ. जगदीश गुप्त। (जन्म 1924; निधन 2001.)
प्रमुख कृतियाँ : नाँव के पाँव, शब्द दंश, हिम विद्ध, गोपा-गौतम (काव्य संग्रह), ‘शंबूक’ (खंडकाव्य), भारतीय कला के पदचिह्न,
नयी कविता : स्वरूप और समस्याएँ, केशवदास (आलोचनाएँ) तथा ‘नयी कविता’ (पत्रिका) आदि।
विशेषता : प्रयोगवाद के बाद जिस नयी कविता का प्रारंभ हुआ, उसके प्रवर्तकों में जगदीश गुप्त का नाम प्रमुख रूप से लिया जाता है।
विधा : नई कविता। नए भावबोधों की अभिव्यक्ति के साथ नए मूल्यों और नए शिल्प विधान का अन्वेषण नई कविता की विशेषता है।
![]()
विषय प्रवेश : प्रस्तुत नई कविता में कवि ने संघर्ष करने की प्रेरणा दी है। संघर्ष ही जीवन की सच्चाई है। जो मनुष्य कठिनाइयों और मुसीबतों का सामना करते हुए बिना झुके या रुके आगे बढ़ता रहता है, वही सच्चा मनुष्य है। जिंदगी लीक से हटकर चलने का नाम है। लीक से भटककर भी मंजिल अवश्य मिलती है। कवि का कहना है कि हमें अपनी समस्याएँ खुद सुलझानी होंगी। हमारी लड़ाई – कोई दूसरा लड़ने नहीं आएगा। हमें खुद योद्धा बनकर अपनी लड़ाई लड़नी है।
सच हम नहीं; सच तुम नहीं कविता का सरल अर्थ
सच हम नहीं …………………………………………….. है जीवन वही।
कवि कहते हैं कि न मेरी बात सच है और न तुम्हारी बात सच है। सच है तो निरंतर संघर्ष करना। संघर्ष ही जीवन है। हमें संघर्ष का रास्ता अपनाना चाहिए। कवि के अनुसार संघर्ष से हटकर जीने की बात ही नहीं करनी चाहिए। बिना संघर्ष का जीवन भी भला कोई जीवन है!
कवि कहते हैं कि जिसने अधीनता स्वीकार ली, वह मृतक के समान हो गया। उसकी हालत डाल से झड़े हुए फूल जैसी होती है। जो व्यक्ति संघर्ष के मार्ग पर चलता हआ भटक जाने पर भी अपनी मंजिल पर बढ़ने से नहीं रुका अथवा अपने प्रयास में असफल हो जाने पर भी जिसने हार नहीं मानी अथवा जिसने मृत्यु से भी मोर्चा लिया हो और उसको परास्त कर दिया हो, उसी का जीवन जीवन कहलाने के योग्य है। यही सच्चाई है।

ऐसा करो जिससे …………………………………………….. यौवन का यही।
कवि कहते हैं कि मनुष्य में कहीं भी कोई ठहराव नहीं आना चाहिए। जो जहाँ है उसे वहीं चुपचाप अपना संघर्ष जारी रखना चाहिए। वे कहते हैं कि परिस्थितियाँ कैसी भी हों चाहे प्रतिकूल स्थिति हो अथवा अनुकूल स्थिति, मनुष्य को हताश होकर अपना संघर्ष कभी भी त्यागना नहीं चाहिए। जीवन का यही संदेश है।
हमने रचा जाओ …………………………………………….. पानी-सी बही।
कवि कहते हैं कि यथास्थिति में जीने का हमने जो नियम बनाया था, आओ, अब हम उसे तोड़ दें। यह भी कोई जीवन है। जीवन तो वह है, जो मँझधार को भी मोड़ने की शक्ति रखता हो। जिसने संघर्ष किया ही नहीं और यथास्थिति को ही सुखमय मानकर जीवन जीता आ रहा हो और दूसरों के इशारों पर चलता आ रहा हो, उसकी भला कोई जिंदगी है? वह जिंदगी तो यथास्थिति को स्वीकार लेने और लीक पर चलनेवाला जीवन है। (इसमें संघर्ष का नामोनिशान नहीं है।)
अपने हृदय का …………………………………………….. दिशा मिलती रही।
कवि कहते हैं कि हमें अपने दुखों को पहचानना होगा। उन्हें दूर करने के लिए हमें स्वयं प्रयास करना होगा। अपनी आँखों के आँसू हमें खुद पोंछने होंगे। हमें अपनी सहायता के लिए किसी अन्य से आशा नहीं करनी है। किसी अन्य की कृपा का भरोसा करना व्यर्थ है। हमें खुद योद्धा बनना होगा। हर संघर्ष करने वाले को कोई-नकोई मार्ग अवश्य मिलता है। मनुष्य मार्ग भटकने के बाद अपने लक्ष्य पर अवश्य पहुँचता है, इस बात को हमें गाँठ बाँध लेनी चाहिए।
![]()
बेकार है मुस्कान से …………………………………………….. राह को ही मैं सही।
कवि कहते हैं कि हृदय के कष्ट को बाह्य मुस्कान से दबाया नहीं जा सकता। इस तरह के प्रयास का कोई लाभ नहीं होता। इसे आदर्श नहीं माना जा सकता है। मनुष्य को भीतर और बाहर दोनों से एक-सा ही रहना चाहिए, यही आदर्श है। कवि कहते हैं कि जब तक विचारों पर अंकुश लगा रहेगा और जब तक प्यार पर दुख की गहरी छाया बनी रहेगी, तब तक इस मार्ग को किसी भी कीमत पर उचित नहीं माना जा सकता।
सच हम नहीं; सच तुम नहीं शब्दार्थ
- नत = झुका हुआ
- जड़ता = अचलता, ठहराव
- पसीजना = मन में दया भाव आना
- वृंत = डंठल
- मँझधार = नदी की बीच की धारा
- चेतना = जागृत अवस्था