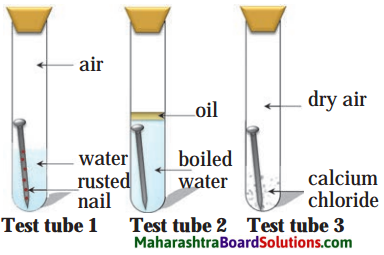
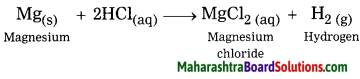
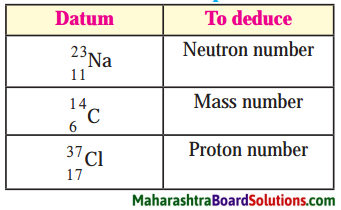
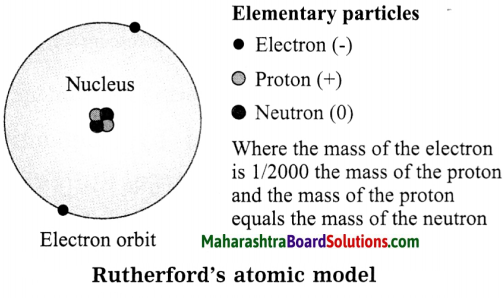
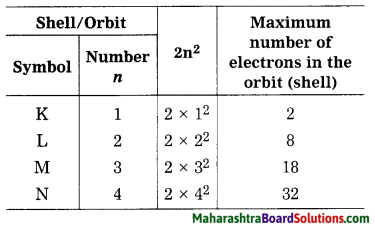
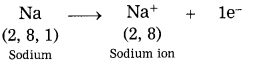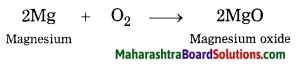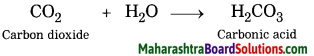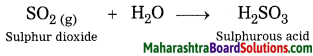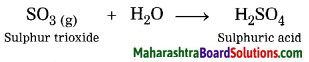Balbharti Maharashtra State Board Class 8 Science Solutions Chapter 13 Chemical Change and Chemical Bond Notes, Textbook Exercise Important Questions and Answers.
Maharashtra State Board Class 8 Science Solutions Chapter 13 Chemical Change and Chemical Bond
Class 8 Science Chapter 13 Chemical Change and Chemical Bond Textbook Questions and Answers
1. Complete the statement by filling the gaps using appropriate term from the terms given in the brackets:
(slow, coloured, arrow, fast, smell, milky, physical, product, chemical, reactant, covalent, ionic, octet, duplet, exchange, sharing, equality sign)
Question a.
An ……….. is drawn in between the reactants and products while writing the equation for a chemical reaction.
Answer:
An arrow is drawn in between the reactants and products while writing the equation for a chemical reaction.
Question b.
Rusting of iron is a ……….. chemical change.
Answer:
Rusting of iron is a slow chemical change.
![]()
Question c.
The spoiling of food is a chemical change which is recognized from the generation of certain …………. due to it.
Answer:
The spoiling of food is a chemical change which is recognized from the generation of certain smell due to it.
Question d.
A colourless solution of calcium hydroxide in a test tube turns ………….. on blowing in it through a blow tube for some time.
Answer:
A colourless solution of calcium hydroxide in a test tube turns milky on blowing in it through a blow tube for some time.
Question e.
The white particles of baking soda disappear when put in lemon juice. This means that it is a ……….. change.
Answer:
The white particles of baking soda disappear when put in lemon juice. This means that it is a chemical change.
![]()
Question f.
Oxygen is a …………….. in respiration.
Answer:
Oxygen is a reactant in respiration.
Question g.
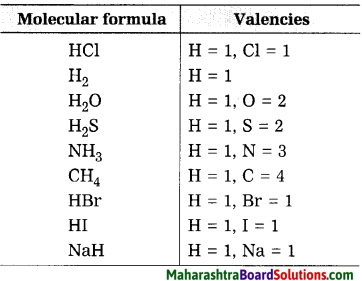
Sodium chloride is …………… compound while hydrogen chloride is compound.
Answer:
Sodium chloride is ionic compound while hydrogen chloride is covalent compound.
Question h.
Electron …………….. is complete in each hydrogen in a hydrogen molecule.
Answer:
Electron duplet is complete in each hydrogen in a hydrogen molecule.
Question i.
Chlorine (Cl2) molecule is formed by ………….. of electrons between two chlorine atoms.
Answer:
Chlorine (Cl2) molecule is formed by sharing of electrons between two chlorine atoms.
2. Explain by writing a word equation.
Question a.
Respiration is a chemical change.
Answer:
Respiration is a biological process, in this process air is inhaled, oxygen present in this inhaled air reacts with glucose present in the cells of the body forming carbon dioxide and water. Moreover, we cannot obtain glucose and oxygen from carbon dioxide and water. Hence, respiration is a chemical change.
Word equation:
![]()
Question b.
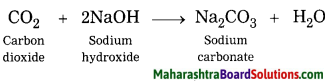
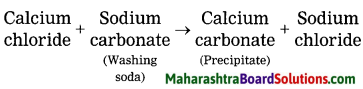
Hard water gets softened on mixing with a solution of washing soda.
Answer:
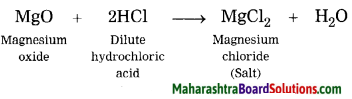
Hard water does not form lather with soap and is brackish to taste. This is because hard water contains the chloride and sulphate salts of calcium and magnesium in dissolved state. When a solution of washing soda is added to hard water, it forms a precipitate of calcium carbonate and magnesium carbonate, which is removed by filtration thus water is softened.
Word equation:
Question c.
Limestone powder disappears on adding to dilute hydrochloric acids.
Answer:
In the reaction of dil. HCl and limestone powder (CaCO3), limestone disappears slowly and carbon dioxide (CO2) liberates slowly.
Word equation:
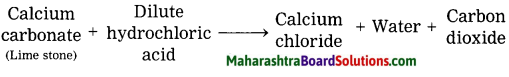
Question d.
Bubbles are seen on adding lemon juice to baking soda.
Answer:
When baking soda is added to lemon juice a chemical change takes place in citric acid present in the lemon juice and carbon dioxide gas is formed. Word equation:

This is a neutralization reaction.
3. Match the pairs.
Question a.
| Column I | Column II |
| 1. Photosynthesis | a. Tendency to lose electrons |
| 2. Water | b. Reactant in combustion process |
| 3. Sodium chloride | c. Chemical change |
| 4. Dissolution of salt in water | d. Covalent bond |
| 5. Carbon | e. Ionic bond |
| 6. Fluorine | f. Physical change |
| 7. Magnesium | g. Tendency to form anion |
Answer:
| Column I | Column II |
| 1. Photosynthesis | c. Chemical change |
| 2. Water | d. Covalent bond |
| 3. Sodium chloride | e. Ionic bond |
| 4. Dissolution of salt in water | f. Physical change |
| 5. Carbon | b. Reactant in combustion process |
| 6. Fluorine | g. Tendency to form anion |
| 7. Magnesium | a. Tendency to lose electrons |
![]()
4. Show with the help of diagram of electronic configuration how the following compounds are formed from the constituent atoms.
Question a.
Sodium Chloride:
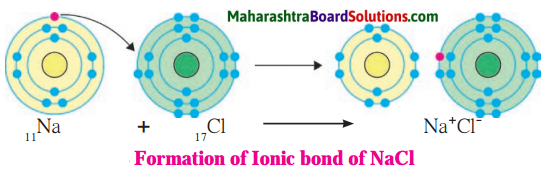
Answer:
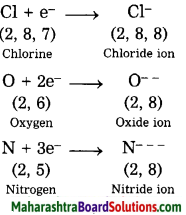
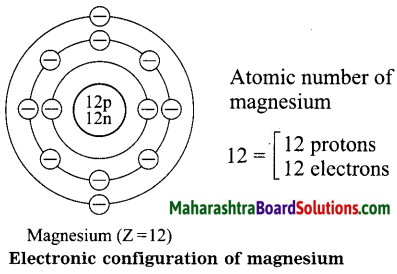
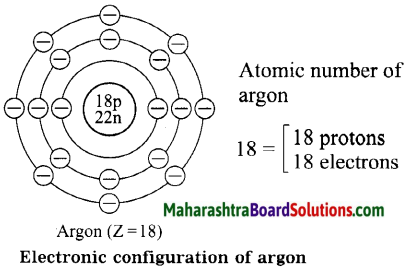
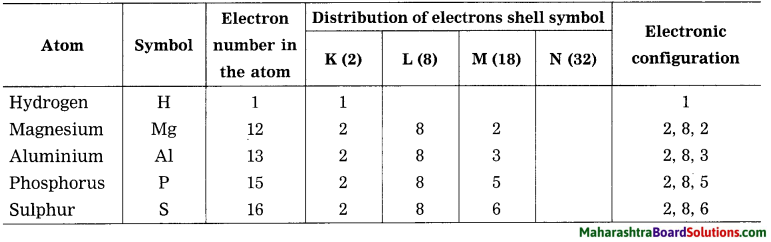
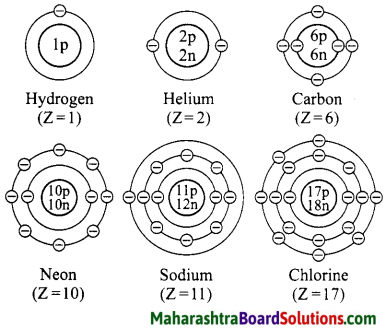
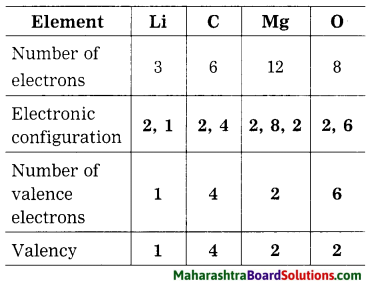
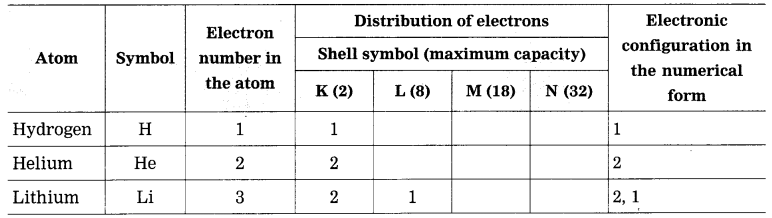
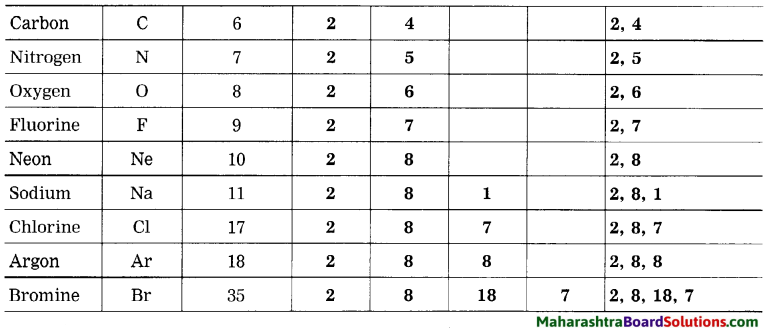
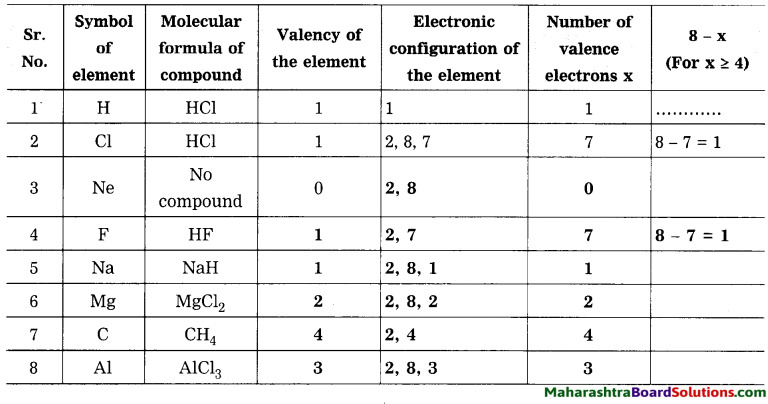
1. Sodium has atomic number 11 and electronic configuration 2, 8, 1.
2. Sodium atom has 1 electron in its outermost shell.
3. It loses one electron from its outermost shell, i.e., M shell. Then its L shell becomes the outermost shell with a stable octet. The nucleus of sodium atom has 11 protons but the number of electrons in the atom has become 10. So, there is a net unit positive charge giving a sodium cation (Na+).
4. On the other hand, chlorine has electronic configuration 2, 8, 7. Chlorine atom has 7 electrons in its outermost shell and requires one electron to complete its octet.
5. Thus, the electron lost by sodium is taken up by chlorine.
6. When chlorine atom gains one electron, octet of chlorine is completed and its K, L, M shells have together 18 electrons and the nucleus has 17 protons. This leads to the formation of an ion (CP).
7. Thus, a chlorine atom accepts one electron from a sodium atom and consequently a chloride ion with one unit negative charge and a sodium ion with one unit positive charge are formed.
8. Sodium and chloride ions, being oppositely charged, attract each other due to the electrostatic force of attraction. An ionic bond is formed and this results in the formation of sodium chloride (NaCl) molecule.
![]()
Question b.
Potassium fluoride:
Answer:
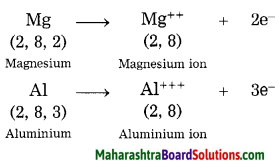
1. Potassium has atomic number 19 and electronic configuration 2, 8, 8, 1.
2. Potassium atom has 1 electron in its outermost shell.
3. It loses one electron from its outermost shell, i.e. N shell. Then its M shell becomes the outermost shell with a stable octet. The nucleus of potassium atom has 19 protons but the number of electrons in the atom has become 18. So there is a net unit positive charge giving a potassium cation (K+).
4. On the other hand, fluorine has electronic configuration 2, 7. Fluorine has 7 electrons in the outermost shell and requires one electron to complete its octet.
5. Thus, the electron lost by potassium is taken up by chlorine.
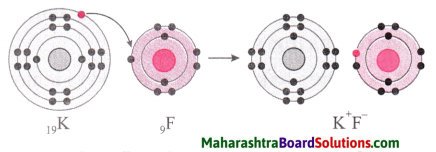
6. When fluorine atom gains one electron, octet of fluorine is completed, its K and L shells have together 10 electrons and the nucleus has 9 protons. This leads to the formation of an ion (F–).
7. Thus, a fluorine atom accepts one electron from a potassium atom and consequently a fluoride ion with one unit negative charge and a potassium ion with one unit positive charge are formed.
8. Potassium and fluoride ions, being oppositely charged, attract each other due to electrostatic force of attraction. An ionic bond is formed and this results in the formation of potassium fluoride (KF) molecule.
![]()
Question c.
Water:
Answer:
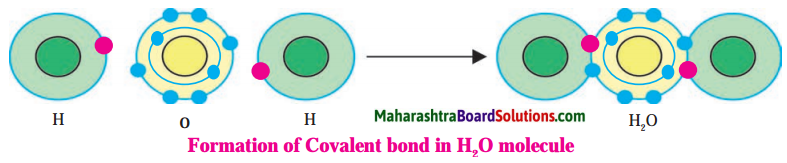
1. Hydrogen has atomic number 1 and electronic configuration 1.
2. Hydrogen has 1 electron in its K shell.
3. Oxygen has atomic 8 and electronic configuration 2, 6. There are 6 electrons in the valence shell of oxygen atom. It means that the electron octet in oxygen is short of two electrons and the valency of oxygen is two.
4. In the H2O molecule, the oxygen atom complete its octet by sharing two electrons one each with two hydrogen atoms, thus, forming two covalent bonds. While this happens, the duplets of two hydrogen atoms are completed.
![]()
Question d.
Hydrogen chloride:
Answer:
1. Hydrogen has atomic number 1 and electronic configuration 1, that means it has 1 electron in its K shell and its duplet is short of one electron therefore, the valency of hydrogen is one.
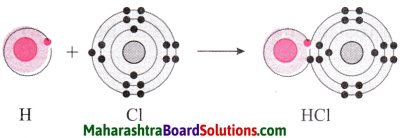
2. On the other hand, chlorine has electronic configuration 2, 8, 7. Chlorine atom has 7 electrons in its outermost shell and requires one electron to complete its octet.
3. The two atoms, hydrogen and chlorine share one electron with each other. As a result, the electron duplet of hydrogen and octet of chlorine is complete and a covalent band is formed between them.
Can you recall?
Question a.
What are the methods of classification of changes?
Answer:
The methods of classification of changes: Physical change and chemical change.
Question b.
What is the difference between physical and chemical change?
Answer:
- In physical change, the composition of substance does not change. No new substance is formed.
- In chemical change, the composition of compounds change and new compounds are formed.
Question c.
Classify the following changes into physical and chemical change. Ripening of mango, melting of ice, boiling of water, dissolution of salt in water, Ripening of banana, fragrance on ripening fruit, darkening of a cut potato, bursting of an inflated balloon, sound of bursting fire cracker, foul smell from a portion of spoiled food.
Answer:
- Physical change: Melting of ice, boiling of water, dissolution of salt in water.
- Chemical change: Ripening of mango, ripening of banana, fragrance of ripening fruit, darkening of a cut potato, bursting of an inflated balloon, sound of bursting firecracker, foul smell from a spoiled food.
![]()
Project:
Question a.
Prepare a list of the chemical changes that occur in your house and surroundings and discuss these in the class.
Class 8 Science Chapter 13 Chemical Change and Chemical Bond Important Questions and Answers
Fill in the blanks:
Question 1.
……………. is a continuously occurring biological process.
Answer:
Respiration is a continuously occurring biological process.
Question 2.
The reaction between citric acid and sodium bicarbonate is a ……………. reaction.
Answer:
The reaction between citric acid and sodium bicarbonate is a neutralization reaction.
Question 3.
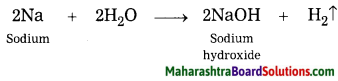
Combustion of fuel is a ………… and ……………. chemical change.
Answer:
Combustion of fuel is a fast and irreversible chemical change.
Question 4
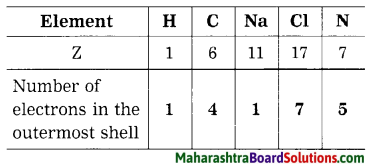
Electronic configuration of sodium is …………….
Answer:
Electronic configuration of sodium is 2, 8, 1.
![]()
Question 5.
Electronic configuration of chlorine is …………….
Answer:
Electronic configuration of chlorine is 2, 8, 7.
Question 6.
A chemical bond formed by sharing of valence electrons of two atoms with each other is called a …………….
Answer:
A chemical bond formed by sharing of valence electrons of two atoms with each other is called a covalent bond.
Question 7.
Hard water contains the chloride and sulphate salts of ……………. and ……………. in dissolved state.
Answer:
Hard water contains the chloride and sulphate salts of calcium and magnesium in dissolved state.
Question 8.
Electronic configuration of fluorine is …………….
Answer:
Electronic configuration of fluorine is 2, 7.
Question 9.
Green plants perform ……………. in sunlight.
Answer:
Green plants perform photosynthesis in sunlight.
Question 10.
Melting of ice is a ……………. change.
Answer:
Melting of ice is a physical change.
Question 11.
The chloride ion has a ……………. charge.
Answer:
The chloride ion has a negative charge.
![]()
Question 12.
A covalent bond between two atoms is also represented by ……………. joining their symbols.
Answer:
A covalent bond between two atoms is also represented by dash joining their symbols.
Rewrite the following statements by selecting the correct options:
Question 1.
Sodium atoms and sodium ions ………….. .
(a) are chemically the same
(b) have the same number of protons
(c) have the same number of electrons
(d) form covalent bond
Answer:
(b) have the same number of protons
Question 2.
An ionic bond is formed when …………….. .
(a) two metallic elements react
(b) two nonmetallic elements react
(c) a metallic element reacts with a non-metallic element
(d) a pair of elements react
Answer:
(c) a metallic element reacts with a non-metallic element
![]()
Question 3.
…………….. tries to establish the duplet state in its outermost orbit by sharing electrons.
(a) Sodium
(b) Potassium
(c) Hydrogen
(d) Magnesium
Answer:
(c) Hydrogen
Question 4.
…………… is an electron donor.
(a) Helium
(b) Iodine
(c) Chlorine
(d) Magnesium
Answer:
(d) Magnesium
Question 5.
………………. combine to form an ionic compound.
(a) Hydrogen and chlorine
(b) Hydrogen and oxygen
(c) Potassium and chlorine
(d) Nitrogen and oxygen
Answer:
(c) Potassium and chlorine
![]()
Question 6.
……………. is an example of chemical change.
(a) Magnetism of iron
(b) Rusting of iron
(c) Heating of iron till it becomes red hot
(d) Dissolution of salt in water
Answer:
(b) Rusting of iron.
State whether the following statements are True or False. (If a statement is false, correct it and rewrite it.)
Question 1.
The preparation of cold drink soda- lemon is a physical change.
Answer:
False. [The preparation of cold drink soda-lemon is a chemical change.]
Question 2.
Hard water contains the chloride and sulphate salts of calcium and magnesium in dissolved state.
Answer:
True.
Question 3.
Combustion of fuel is a fast and irreversible man-made chemical change.
Answer:
True.
Question 4.
Photosynthesis reaction is a man-made chemical change.
Answer:
False. [Photosynthesis reaction is a natural chemical change.]
Question 5.
The atoms with incomplete electron octet/duplet form chemical bonds.
Answer:
True.
Question 6.
Electronic configuration of chlorine is 2, 8, 6.
Answer:
False. (Electronic configuration of chlorine is 2, 8, 7.)
Question 7.
One ionic bond is formed due to the electrical change +1 or -1 on an ion.
Answer:
True.
![]()
Question 8.
H2O Molecule is an ionic compound.
Answer:
False. (H2O molecule is a covalent compound.)
Question 9.
The bond between two chlorine atoms is a covalent band.
Answer:
True.
Question 10.
Arrow indicates the direction of the reaction.
Answer:
True.
Consider the correlation between the words of the first pair, match the third word/words with the most appropriate answer:
Question 1.
K : 2, 8, 8, 1 : : Mg : ………………
Answer:
2, 8, 2
Question 2.
MgCl2 : Ionic bond : : CaO : ……………..
Answer:
Covalent bond
![]()
Question 3.
Photosynthesis : Natural chemical change : : Cold drink, soda lemon : ………
Answer:
Man-made chemical change
Question 4.
Respiration: Glucose + Oxygen : : Neutralization : ………….
Answer:
Acid + alkali.
Match the columns:
Question 1.
| Column I | Column II |
| 1. Respiration | a. Potassium and fluorine |
| 2. Acid + Base | b. Glucose and oxygen |
| 3. Photosynthesis | c. Carbon dioxide and water |
| 4. Ionic bond | d. Salt and water |
Answer:
| Column I | Column II |
| 1. Respiration | c. Carbon dioxide and water |
| 2. Acid + Base | d. Salt and water |
| 3. Photosynthesis | b. Glucose and oxygen |
| 4. Ionic bond | a. Potassium and fluorine |
![]()
Define the following:
- Chemical change: In a chemical change, the chemical composition of the original matter changes and new substances having different properties and different chemical composition are formed.
- Ionic bond: The chemical bond formed due to an electrostatic force of attraction between the oppositely charged cation and anion is called an ionic bond or an electrovalent bond.
- Covalent bond: The chemical bond formed by sharing of valence electrons of two atoms with each other is called a covalent bond.
Answer the following questions in one sentence each:
Question 1.
Name the reactants in respiration.
Answer:
Glucose and oxygen are the reactants in respiration.
Question 2.
Name the products of respiration.
Answer:
Carbon dioxide and water are the products of respiration.
![]()
Question 3.
Name the reactants in photosynthesis.
Answer:
Carbon dioxide and water are the reactants in photosynthesis.
Question 4.
Name the products of photosynthesis.
Answer:
Glucose and oxygen are the products of photosynthesis.
Question 5.
Name the salts present in hard water.
Answer:
Salts present in hard water are calcium chloride, magnesium chloride, calcium sulphate and magnesium sulphate.
Question 6.
Give two examples of ionic compounds.
Answer:
Ionic compounds: Sodium chloride (NaCl), Potassium fluoride (KF).
Question 7.
Give two examples of covalent compounds.
Answer:
Covalent compounds: Hydrogen (H2), Water (H2O).
![]()
Question 8.
Name an acid used for cleaning Shahabad tile.
Answer:
An acid used for cleaning Shahabad tile is dilute hydrochloric acid (HCl).
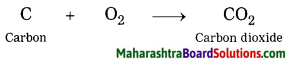
Question 9.
Write a chemical equation for combustion of fuel.
Answer:
Chemical equation:
C + O2 → CO2
Question 10.
Write a chemical equation for photosynthesis.
Answer:
Chemical equation:
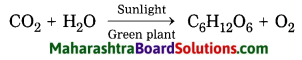
Answer the following questions:
1. Explain by writing a word equation.
Question 1.
Combustion of fuel is a fast and irreversible chemical change.
Answer:
Wood, coal, petrol or cooking gas are burnt for producing energy. Carbon is the common substance that burns in all these fuels. The product carbon dioxide is formed when carbon combines with oxygen in the air during the combustion process. We cannot obtain fuel from carbon dioxide by employing any other method. Properties of carbon dioxide are altogether different from those of fuel. Hence, this change is a irreversible chemical change.
Word equation:
Carbon + Oxygen → Carbon dioxide
![]()
Question 2.
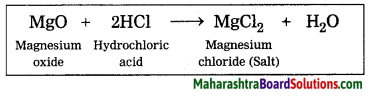
Dilute hydrochloric acid is used for cleaning Shahabad tiles.
Answer:
The main constituent of Shahabad tile is calcium carbonate. During its cleaning with hydrochloric acid, the upper layer of the tile reacts with hydrochloric acid and three products are formed. One of them is calcium chloride, which being soluble in water, gets washed away with water. The second product is carbon dioxide, it mixes with air. The third product is water.
Word equation:
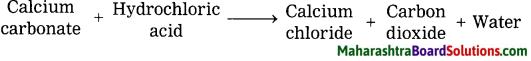
2. Write a chemical equation (unbalanced) for the following reactions:
Question 1.
Answer:
Chemical equation:
CO2 + Ca(OH)2 → CaCO3 + H2O
Question 2.
Answer:
Chemical equation:
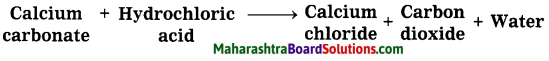
CaCO3 + HCl → CaCl2 + CO2 + H2O
Question 3.
Magnesium salts during the softening of hard water.
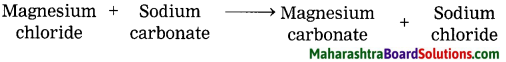
Answer:
Chemical equation:
MgCl2 + Na2CO3 → MgCOg + 2NaCl
3. Show with the help of diagram of electronic configuration how the following compounds are formed from the constituent atoms.
Question 1.
Hydrogen molecule:
Answer:
1. Hydrogen has atomic number 1 and electronic configuration 1. That means it has 1 electron in its K shell and its duplet is short of one electron therefore, the valency of hydrogen is one.
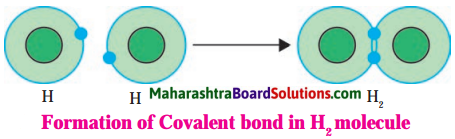
2. The two atoms of hydrogen are identical, they share their electrons with each other.
3. As a result, the electron duplet of both the hydrogen atoms is complete and a covalent band is formed between them.
![]()
4. There is one covalent bond between the component atoms H and Cl of the molecule HCl. Use this information to represent the formation of HCl molecules from H and Cl atoms diagrammatically.
(Use your brainpower)
Answer:
Hydrogen chloride:
1. Hydrogen has atomic number 1 and electronic configuration 1, that means it has 1 electron in its K shell and its duplet is short of one electron therefore, the valency of hydrogen is one.

2. On the other hand, chlorine has electronic configuration 2, 8, 7. Chlorine atom has 7 electrons in its outermost shell and requires one electron to complete its octet.
3. The two atoms, hydrogen and chlorine share one electron with each other. As a result, the electron duplet of hydrogen and octet of chlorine is complete and a covalent band is formed between them.
5. Show the formation of the following ionic compounds from the corresponding elements using two methods namely, numerical and diagrammatic representation of electronic configuration.
(a) K+ F–, from 19K and 9F, (b) Ca2+ O2- from 20Ca and 8O.
(Use your brainpower)
Answer:
(a) K+F– from 19K and 9F
Potassium fluoride:
1. Potassium has atomic number 19 and electronic configuration 2, 8, 8, 1.
2. Potassium atom has 1 electron in its outermost shell.
3. It loses one electron from its outermost shell, i.e. N shell. Then its M shell becomes the outermost shell with a stable octet. The nucleus of potassium atom has 19 protons but the number of electrons in the atom has become 18. So there is a net unit positive charge giving a potassium cation (K+).
4. On the other hand, fluorine has electronic configuration 2, 7. Fluorine has 7 electrons in the outermost shell and requires one electron to complete its octet.
5. Thus, the electron lost by potassium is taken up by chlorine.

6. When fluorine atom gains one electron, octet of fluorine is completed, its K and L shells have together 10 electrons and the nucleus has 9 protons. This leads to the formation of an ion (F–).
7. Thus, a fluorine atom accepts one electron from a potassium atom and consequently a fluoride ion with one unit negative charge and a potassium ion with one unit positive charge are formed.
8. Potassium and fluoride ions, being oppositely charged, attract each other due to electrostatic force of attraction. An ionic bond is formed and this results in the formation of potassium fluoride (KF) molecule.
(b) Ca2+O2- from 20Ca and 8O.
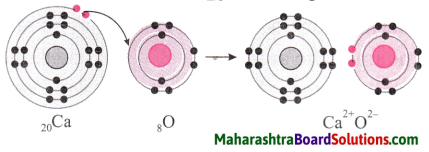
Distinguish between the following:
Question 1.
Physical change and Chemical change:
Answer:
| Physical change | Chemical change |
| 1. In this change, the composition of the substance does not change. No new substance is formed. | 1. In this change, the composition of the compounds change and new compounds are formed. |
| 2. In this case, physical properties such as state, colour, density, etc. are changed. | 2. In this case, physical and chemical properties are entirely changed. |
| 3. This change is temporary. | 3. This change is permanent. |
| 4. In this case, the original substance can be recovered by simple means or by merely reversing the process. | 4. In this case, the original substance cannot be recovered by easy means or by reversing the process. |
![]()
Question 2.
Ionic bond and Covalent bond:
Answer:
| Ionic bond | Covalent bond |
| 1. Ionic bond is formed due to the transfer of electrons from one atom to another. | 1. Covalent bond is formed due to the sharing of electrons between two or more atoms. |
| 2. Atoms of metals and nonmetals combine to form ionic bonds. | 2. Atoms of nonmetals combine to form covalent bonds. |
| 3. Molecules of the compounds formed due to ionic bond split up into ions in aqueous solution. | 3. Molecules of the compounds formed due to covalent bond so not split up into ions in a solution. |
Give scientific reasons:
Question 1.
Ionic compounds are formed due to the combination of metallic and nonmetallic atoms.
Answer:
Metallic atoms have a tendency to lose electrons from their outermost orbits to establish the octet state in their penultimate orbits. Conversely, nonmetallic atoms gain electrons to establish the octet state of their outermost orbits.
When a metallic atom and a nonmetallic atom come close together, the metallic atom loses electrons and gets c converted into positively charged ion, while the nonmetallic’ atom gets converted into negatively charged ions so formed, develop an ionic bond and this results in the formation of an ionic compound. Hence, ionic compounds are formed due to the combination of metallic and nonmetallic atoms.
![]()
Activity-based questions:
Activity 1:

Take the lemon juice in a clean glass. Take two drops of the lemon juice in a spoon and taste. Add a pinch of baking soda in the glass of lemon juice. Did you notice bubbling around the particles of soda? Did you hear a sound on taking your ear near the glass? Now again taste it. Did it taste as sour as it was in the beginning? (Above activity is to be done using clean apparatus and edible material. Then only it is possible to test the ’taste’, otherwise keep in mind that the testing of ‘taste’ cannot be done.)
![]()
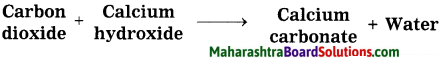
Activity 2:
Take some freshly prepared lime water (solution of calcium hydroxide) in a test tube. Keep on blowing in it with a blow tube. What is seen after some time? Did the colourless lime water turn milky? After some more time you will find that a white insoluble solid settles at the bottom of the test tube. This is a precipitate of calcium carbonate. The turning lime water milky means that the blown gas mixed in it was carbon dioxide.

Write a chemical equation for the above word equation.
Chemical equation:
CO2 + Ca(OH)2 → CaCO3 + H2O