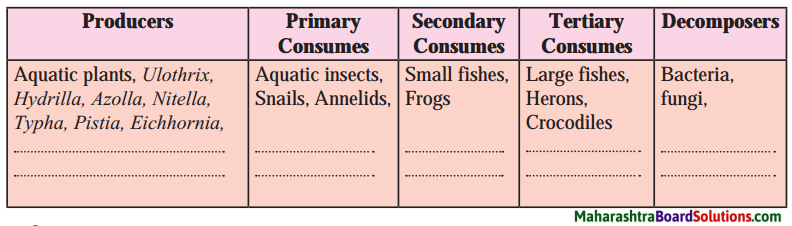
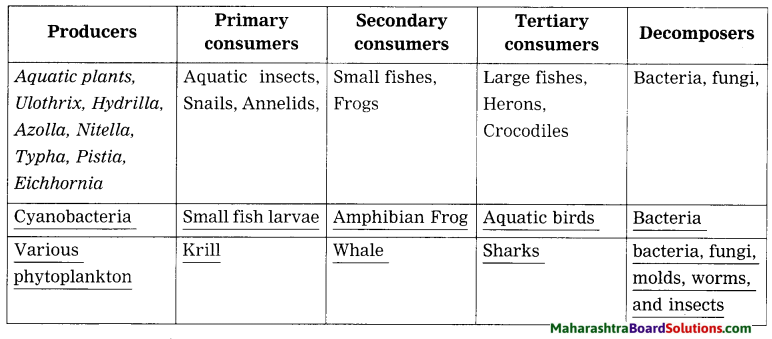
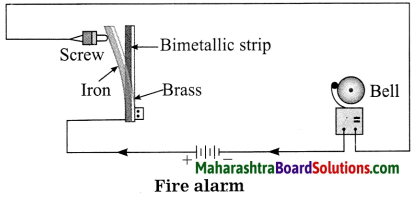
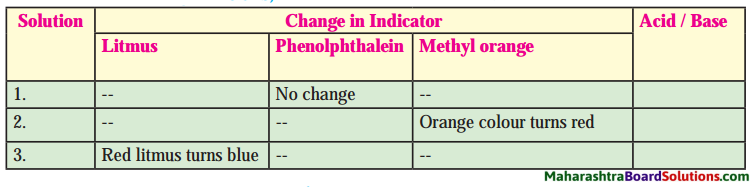
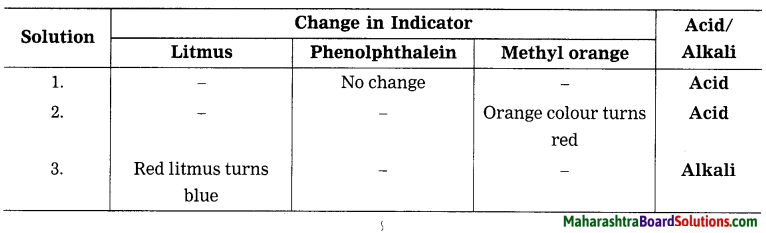
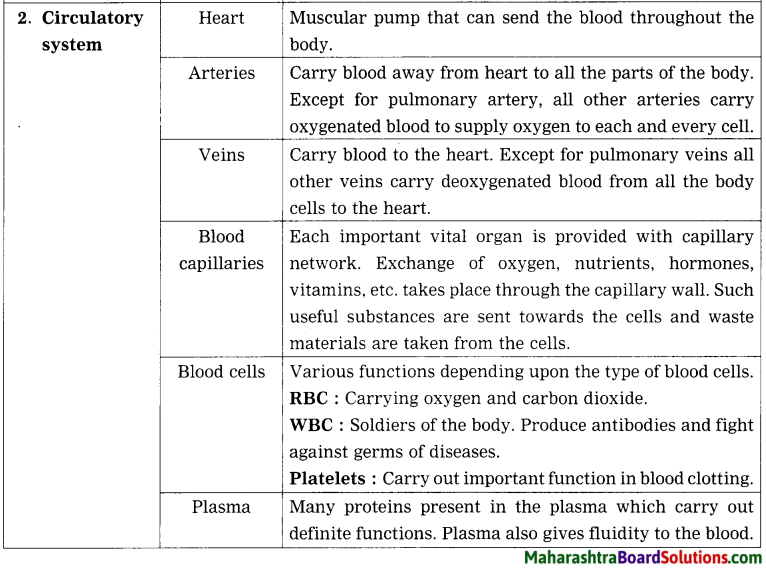
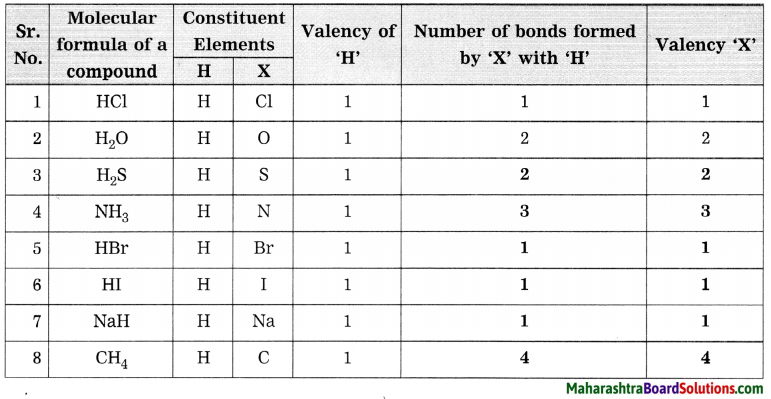
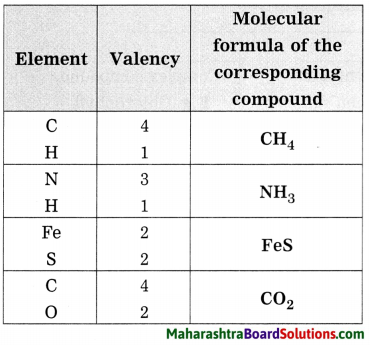
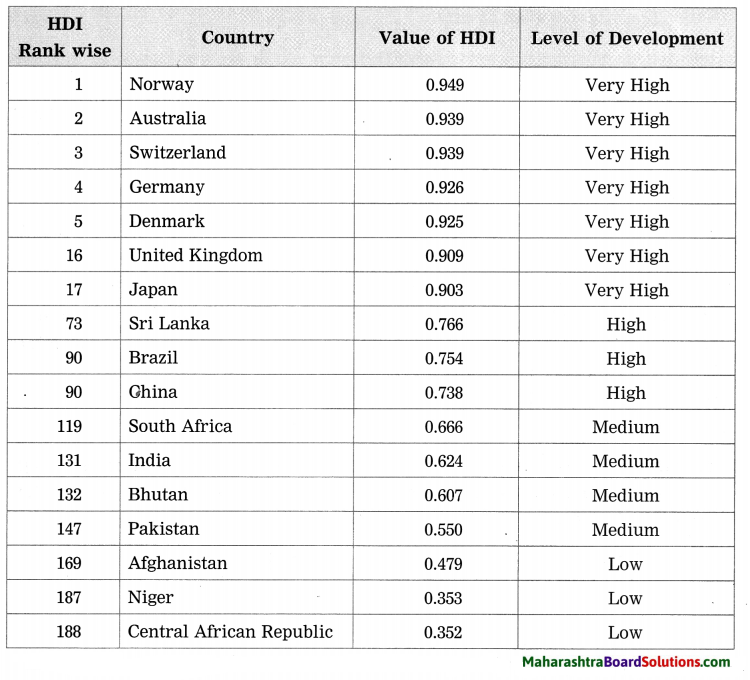
Balbharti Maharashtra State Board Class 8 Science Solutions Chapter 17 Man-made Materials Notes, Textbook Exercise Important Questions and Answers.
Maharashtra State Board Class 8 Science Solutions Chapter 17 Man-made Materials
Class 8 Science Chapter 17 Man-made Materials Textbook Questions and Answers
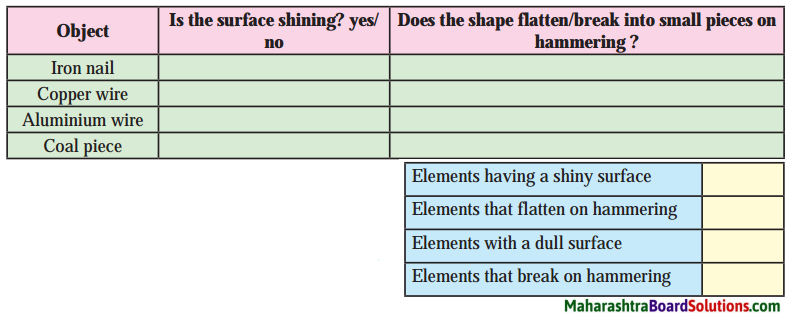
1. Try to find it:
Question a.
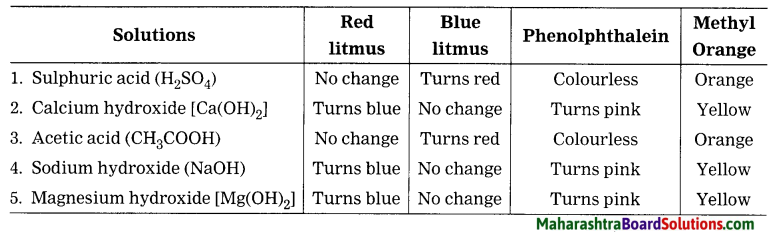
Plastic shows …………. property, hence it can be moulded to any shape.
Answer:
Plastic shows plasticity property, hence it can be moulded to any shape.
Question b.
Motor cars are coated with ……… .
Answer:
Motor cars are coated with Teflon.
Question c.
Thermocol melts at ………… °C.
Answer:
Thermocol melts at more than 100 °C (it is about 240 °C).
Question d.
…………….. glass dissolves in water.
Answer:
Alkali silicate or water glass dissolves in water.
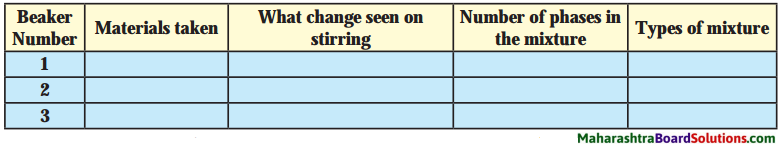
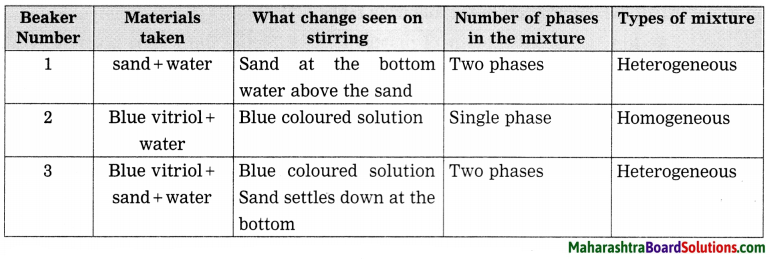
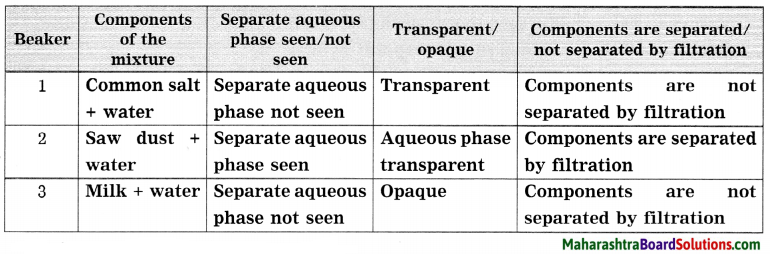
![]()
2. Who is my partner?
Question a.
| Column ‘A’ | Column ‘B’ |
| 1. Lead glass | a. Plates |
| 2. Bakelite | b. Mattresses |
| 3. Thermocol | c. Electric bulb |
| 4. Optic glass | d. Electric switch |
| 5. Polypropylene | e. Lens |
Answer:
| Column ‘A’ | Column ‘B’ |
| 1. Lead glass | c. Electric bulb |
| 2. Bakelite | d. Electric switch |
| 3. Thermocol | a. Plates |
| 4. Optic glass | e. Lens |
| 5. Polypropylene | b. Mattresses |
![]()
3. Answer the following.
Question a.
Thermocol is produced from which material?
Answer:
Thermocol is made from polystyrene which is also a complex thermoplastic substance.
Question b.
Write uses of PVC.
Answer:
PVC or Polyvinyl chloride is used for making bottles, raincoat, pipes, handbags, shoes, electric cable insulation, furniture, ropes, toys, etc.
Question c.
Write the natural or man-made raw material of the following items.
Mattress, beaker, bangle, chair, gunny bag, broom, knife, pen.
Answer:
| Items | Natural raw material | Man-made raw materials |
| Mattress | Cotton, Coir (Jute fibres) | Polypropylene |
| Beaker | ___ | Glass (Silicate or borosilicate glass) |
| Bangle | Gold, silver, lac, copper | Plastic, Glass |
| Chair | Wood | Plastic (PVC) |
| Gunny bag | Jute, cotton | Plastic (PVC) |
| Broom | Plant fibres | Plastic fibres (PVC) |
| Knife | Metals such as iron | Plastic |
| Pen | Metals | Plastic |
![]()
Question d.
Which are the main ingredients of glass?
Answer:
The main ingredients of glass are sand and silica.
Question e.
How the plastic is produced?
Answer:
- Plastics are derived from natural materials such as natural gas, oil, coal, minerals and plants.
- The first synthetic plastics were i derived from cellulose, a substance found in plants and trees. This cellulose was heated with chemicals and resulted in a plastic like material.
- In modern times, the different raw materials are used for making plastics, but most plastics are made from the hydrocarbons present in the natural gas, oil and coal.
- Plastics are simply chains of like molecules linked together. These chains are called polymers. Thus, many plastics begin with “poly,” such as polyethylene, polystyrene and polypropylene.
- These polymers are made of carbon and hydrogen and sometimes oxygen, nitrogen, sulphur, chlorine, fluorine, phosphorous or silicon.
- Plastic is produced in factories by suitable chemical reactions.
![]()
4. Distinguish between.
Question a.
Man-made material and natural material
Answer:
| Man-made material | Natural material |
| 1. The man-made materials are obtained from processes in scientific laboratory. | 1. Natural materials are obtained from nature. |
| 2. Man-made materials are subjected to rigorous processing to alter the material for serving the intended purpose. | 2. Natural materials are subjected to less treatment and processing. |
| 3. Man-made materials are typically much more durable having a very long lifespan. | 3. Natural materials have shorter lifespan, because these materials were once alive and so gradually perish over time. |
| 4. Maintaining man-made materials require less care and attention. | 4. Maintaining natural materials requires lots of care and continuous attention. |
| 5. Man-made materials can have a negative environmental impact because they are not sustainable. E.g. Glass, plastic, Thermocol, soil, metals, rubber. |
5. Natural materials do not have a negative environmental impact because they can be degraded easily. E.g. Cotton, silk, wood. |
![]()
Question b.
Thermoplastic and Thermosetting plastic:
Answer:
| Thermoplastic | Thermosetting plastic |
| 1. The plastic that can be moulded as per our wish is called thermoplastic. | 1. The plastic in which a specific shape is given with the help of mould and this shape cannot be changed again on heating is called thermosetting plastic. |
| 2. Thermoplastic substances can be recycled and reused. E.g. PVC – Polyvinyl chloride, PS – Polystyrene, PE – Polyethylene and PP – Polypropylene are types of thermoplastics. |
2. Thermosetting plastic cannot be reused again. E.g. Bakelite, Melamine, Polyurethane and polyster are the types of thermosetting plastics. |
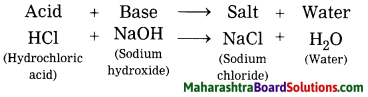
5. Answer the following in your own words.
Question a.
Explain the effect of following materials on environment and human health.
1. Plastic
2. Glass.
3. Thermocol.
Answer:
1. Plastic:
- Plastic is non-degradable substance. Hence if thrown in any ecosystem, it remains unchanged for many years.
- It is one of the worst environmental pollutants as its disposal is a major problem.
- If thrown in water bodies, it affects the aquatic animals. Many of turtles mistake it for algae and eat the plastic. Eventually such animals die due to choking.
- In terrestrial environment, the grazing animals like cattle are affected due to plastic.
- If burnt it emits very toxic gases.
- In landfill sites, it remains unchanged for thousands of years.
2. Glass:
- The glass production is carried out at high temperatures of about 1500 °C. This burning emits many hazardous gases like sulphur dioxide, nitrogen dioxide, carbon dioxide. These gases cause the greenhouse effect.
- Moreover, glass being non-degradable, cause pollution.
- If broken glass pieces or any waste glass material is disposed into aquatic environment, it affects animals and plants.
- Similarly, glass pieces block the drainages.
- The waste glass thrown anywhere cause injury to terrestrial fauna.
3. Thermocol:
- Thermocol contains carcinogenic ingredients in the form of styrene. If there is prolonged contact with thermocol, there is a possibility of blood cancer like leukemia and lymphoma.
- Thermocol is non-degradable. It cannot be degraded into harmless substances easily.
- If it is burnt for destruction, it releases toxic gases in atmosphere.
- The plates and cups used for food, water, tea, etc. are made up of thermocol. This may affect the health. Reheating the food kept in thermocol releases styrene. This styrene may dissolve in that food, causing health problems like cancer.
![]()
Question b.
Which measures will you arrange to minimize the environmental problems arising due to non-degradable plastic?
Answer:
- The use of plastic should be minimum. Reducing the consumption, reusing the same plastic again and again, recycling the used plastic and making some new products from the used plastic are some of the measures that can be adopted.
- There are attempts to use plastic in making roads. Therefore, plastic is bought with good price at some places.
- Therefore, instead of disposing of it anywhere, it should be collected and sold in best possible way.
- The better alternatives for plastic should be adopted.
- The awareness programmes about misuse of plastic should be arranged so that common man can understand the dangers of using plastic.
6. Write short notes.
Question a.
Glass production:
Answer:
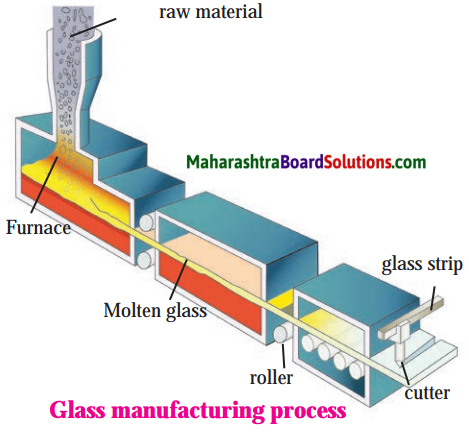
The general preparation of the glass is as follows:
- Mixture of sand, soda, lime and small quantity of magnesium oxide is heated in furnace.
- At 1700 °C sand or silicon dioxide melts.
- To make the mixture melt at lesser temperature, pieces of discarded glasses are added to it.
- This addition makes the mixture to melt at lesser temperature of 850 °C.
- When all the ingredients of mixture are liquified, then again it is heated up to 1500 °C.
- This heating is immediately followed by cooling.
- The sudden cooling causes the mixture to become homogeneous, amorphous and transparent instead of crystalline.
- For variety of glass types, different proportions of ingredients are used for heating.
![]()
Question b.
Optic glass:
Answer:
- Optic glass or optical glass needs to be very clear and transparent as it is used in spectacles, lenses and other devices like microscopes.
- Optic glasses are produced from the mixture of sand, soda, limestone, barium oxide and boron.
Question c.
Uses of plastic:
Answer:
Plastic, the man-made material is used in various forms in modern age. According to the type of plastic, its uses are different.

I. Thermoplastic materials are used for manufacturing following articles:
- Polyvinyl chloride or PVC is used to make bottles, raincoat, pipes, handbags,: shoes, electric cable insulation, furniture, ropes, toys, etc.
- Polystyrene is used in making thermo insulating parts of electric appliances like refrigerators, gears of machines, toys, protective coverings like covers of CD and DVD, etc.
- Polyethylene (PE) plastics are used for making milk bags, packing bags, flexible garden pipes, etc.
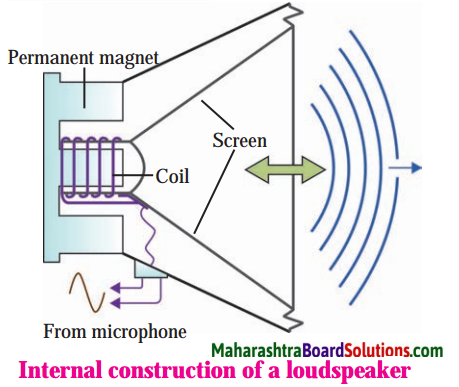
- Polypropylene (PP) is used in making parts of loudspeakers and vehicles, ropes, mattresses, laboratory appliances, etc.
II. Thermosetting plastic is used in the manufacturing of the following items:

- Bakelite for making cabinets of radio, T.V., telephones, electric switches, toys, plastic handles of cookers, etc.
- Melamine for making domestically useful items like cup – saucers, plates, tray, some spare parts of airplane engines, electric and sound insulating coverings, etc.
- Polyurethane in making surfing boards, small boats, furniture, seats in vehicles, etc.
- Polyester in fibreglass, toners of laser printers, textile industry, etc.
![]()
Can you tell?
Question 1.
Make a list of 20 different man-made materials present in your home, school and places around and discuss.
Answer:
Schoolbag, books, computer, table lamp, tube lights, benches, shoes, dress, pencil, pen, tiffin box, water bottle, raincoat, umbrella, medicine tablets, pen drive, chalk, duster, utensils, gas burner, biscuits.
Can you recall?
Question 1.
How many plastic carry bags are brought in your home in a day? What happens to those later on?
Answer:
Till May 2018 about one or two plastic bags were brought in our home every day. But now due to prohibition on plastic bags and other plastic items by the Government, the use of plastic has been drastically reduced. The bags brought at home caused lot of plastic pollution. Plastic is non-biodegradable, thus it always created problem of their disposal.
If dumped in a water body, it can be hazardous for aquatic animals. If thrown in the garbage, plastic articles find their way to landfilling areas. If thrown anyhow plastic may be consumed by stray animals. This causes toxic effects on them. If burnt they emit toxic gases.
Question 2.
How are the used up and thrown away carry bags, water bottles, milk bags recycled?
Answer:
The plastic waste is collected by the kabadiwala or sweepers. They sell these articles to the recycling units. In recycling units, the recycling of some of the plastic items is done. This leads to formation of plastic of low quality which may be used in making some newer plastic items.
![]()
Question 3.
Which material is wrapped around the items of glass or similar material during transport to prevent from breaking up?
Answer:
The plastic bubble wrap or thermocol is used to protect fragile items from the shocks and breaks during their transport.
Project:
Question 1.
Collect the information about the plastic used in production of utensils used in microwave oven.
Question 2.
Collect the information about the material used in production of denture. (A set of artificial teeth)
Class 8 Science Chapter 17 Man-made Materials Important Questions and Answers
Try to find it:
Question 1.
The plastic that can be moulded as per our wish is called …………… .
Answer:
The plastic that can be moulded as per our wish is called thermoplastic.
![]()
Question 2.
…………… is a type of plastic used for manufacturing artificial teeth.
Answer:
Polyacrylic is a type of plastic used for manufacturing artificial teeth.
Question 3.
Thermocol is a form of a complex material called ……………. .
Answer:
Thermocol is a form of a complex material called polystyrene.
Write whether the following statements are True or False: Rewrite the false statements after correcting:
Question 1.
Plastics are inorganic polymers that show plasticity.
Answer:
False. (Plastics are organic polymers that show plasticity.)
Question 2.
Polythene, PVC are thermosetting plastic materials.
Answer:
False. (Polythene, PVC are thermoplastic materials.)
![]()
Question 3.
Plastic is bad conductor of heat and electricity.
Answer:
True.
Question 4.
Surfing boards are made up of bakelite.
Answer:
False. (Surfing boards are made up of polyurethane.)
Question 5.
Being bad conductor of electricity, glass is used as insulator in electric appliances.
Answer:
True.
Match the columns:
Question 1.
| Column A | Column B |
| 1. Borosilicate glass | a. Oxide of specific metal. |
| 2. Alkali silicate glass | b. Sand, soda, limestone, barium oxide and boron. |
| 3. Lead glass | c. Sand and soda. |
| 4. Optical glass | d. Sand, soda, limestone and lead oxide. |
| 5. Coloured glass | e. Sand, soda, boric acid and aluminium oxide. |
Answer:
| Column A | Column B |
| 1. Borosilicate glass | e. Sand, soda, boric acid and aluminium oxide. |
| 2. Alkali silicate glass | c. Sand and soda. |
| 3. Lead glass | d. Sand, soda, limestone and lead oxide. |
| 4. Optical glass | b. Sand, soda, limestone, barium oxide and boron. |
| 5. Coloured glass | a. Oxide of specific metal. |
![]()
Find the odd one out:
Question 1.
Bakelite, Melamine, Polystyrene, Polyester.
Answer:
Polystyrene (All others are thermosetting plastic materials, polystyrene is thermoplastic.)
Question 2.
Polyvinyl chloride, Polystyrene, Polypropylene, Polyurethane.
Answer:
Polyurethane (All others are thermoplastic materials, polyurethane is thermosetting plastic.)
Question 3.
Vegetable peels, Cotton bolls, Wooden scraps, Plastic bag.
Answer:
Plastic bag (All others are degradable materials, plastic is non-degradable.)
![]()
Question 4.
Teflon, Polyacrylic, Fibreglass, Polyester.
Answer:
Fibreglass (All others are types of plastic.)
Considering the relationship in the first pair, complete the second pair:
Question 1.
Processed glass: Fen glass : : Alkali silicate glass : ………..
Answer:
Water glass
Question 2.
Ferrous oxide: Bluish green glass : : Copper oxide : ……..
Answer:
Red glass
Question 3.
Lens : Optical glass : : Laboratory glassware : ……….
Answer:
Silica glass
Question 4.
Light bulbs, Tubes: Lead glass : : Medicine storing : ………
Answer:
Borosilicate glass
![]()
Question 5.
Glass: Mixture of silica and silicate : : Thermocol : ………..
Answer:
Polystyrene.
Distinguish between:
Question 1.
Degradable and Non-degradable substances:
Answer:
| Degradable substances | Non-degradable substances |
| 1. The substances which can be naturally reduced into their inorganic constituents are called degradable substances. | 1. The substances that cannot be degraded on their own into their inorganic constituents are called non-degradable substances. |
| 2. Degradable substances are not accumulated in the nature. | 2. Non-degradable substances remain accumulated in the nature for a long time. |
| 3. Degradable substances emit foul odour when they are being decomposed. | 3. Non-degradable substances may not emit foul odour as they are not degraded. But they make the place look dirty. |
| 4. Usually microorganisms play a part in the degradation process, hence such substances are also called biodegradable. E.g. Vegetables, fruits, wood, cotton or wool fibres, etc. |
4. Microorganisms cannot act on non-degradable substances and hence they are also called non-biodegradable substances. E.g. Plastic, thermocol, glass, metals, etc. |
![]()
Write short notes:
Question 1.
Properties of plastic:
Answer:
- Plastic is non-corrosive.
- It is non-degradable as it does not decompose.
- The factors such as humidity, heat, rain, etc. do not affect plastic.
- Any coloured item can be manufactured from plastic.
- Plastic shows property of plasticity. Thus it can be moulded into any shape that is needed.
- Plastic is a bad conductor of heat and electricity.
- Plastic is light-weight and thus preferred for carrying.
![]()
Question 2.
Properties of Glass:
Answer:
The glass becomes soft on heating and thus can be moulded into desired shape.
- According to ingredients added at the time of preparation of glass, its density changes accordingly.
- Glass is slow conductor of heat. If a cold glass is quickly heated, it cracks suddenly. Similarly, the warm glass if exposed to sudden cooling, it too cracks.
- Glass is a bad conductor of electricity. Therefore, it is used as insulating material in electric appliances.
- Glass is transparent, allowing most of light to pass through it. If there are chromium, vanadium or iron oxides in the glass, large amount of light is absorbed in glass.
Answer the following questions in one sentence only:
Question 1.
What is the use of plastic in healthcare sector?
Answer:
Saline bottles, pouches, blood bags, syringes and medicine bottles are made up of plastic, making plastic irreplaceable in the healthcare sector.
Question 2.
Which material is used in coating of vehicles? Why?
Answer:
Teflon is used for coating the vehicle as it protects the vehicles from scratches.
Question 3.
What is the use of polyacrylic type of plastic?
Answer:
Polyacrylic plastic used for manufacturing lenses and in the manufacture of artificial teeth.
![]()
Question 4.
What is 4R principle?
Answer:
The 4R principle is the way of behaviour that prevents or lessens the environmental pollution. The 4Rs are Reduce, Reuse, Recycle and Recover.
Question 5.
What are the eco-friendly options for a plastic bag?
Answer:
Jute bag, cloth bag and reusable bags of any natural materials are the eco-friendly options for the plastic bag.
Question 6.
How is Borosilicate glass produced?
Answer:
Borosilicate glass is produced by melting the mixture of sand, soda, boric acid and aluminium oxide.
Question 7.
When is large amount of light absorbed in the glass?
Answer:
If there are oxides of either chromium, vanadium or iron in the glass, large amount of light is absorbed in it.
Give scientific reasons:
Question 1.
It is harmful to eat food kept in thermocol.
Answer:
Thermocol is made up from polystyrene. It is carcinogenic (cancer causing) substance. When food kept in r thermocol containers is reheated, this , styrene dissolves in it. This may affect the r health of the person who consumes such food. It has been noted that there is a great possibility of blood cancer like leukaemia r and lymphoma if one remains in contact with thermocol for a longer time. Thus, it is harmful to eat food kept in thermocol.
![]()
Question 2.
Vessels used to cook food in the microwave oven are made up of plastic.
Answer:
The vessels made from metals cannot be used in the microwave oven. The microwaves cannot penetrate steel and so ‘ can only heat the contents through the open top. Some metals cause sparking with microwaves. Microwave energy is absorbed differently by different materials. Plastics of only certain kind are more suitable to be used in the microwave oven.
Answer the following:
Question 1.
What are the qualities of thermocol?
Answer:
- Thermocol is modern man-made material made from polystyrene.
- It can be transformed into liquid state when heated at more than 100 °C temperature. On cooling, it returns to the solid state.
- Therefore, any desired shape can be given to it.
- It acts as a good shock-absorber and hence used as a packing material to transport brittle material.
- It is a bad insulator of heat and hence used for storing fish and other perishable articles in market.
Think about it:
Question 1.
Why are the plastic tanks used for storage of chemicals?
Answer:
Plastic is non-corrosive. It is comparatively non-reactive and it does not decompose. Moreover, the plastic containers are easier for handling. They are lighter and unbreakable. Hence the plastic tanks are used for storage of chemicals.
![]()
Question 2.
Why most of domestically useful items are replaced by plastic?
Answer:
Plastic is lighter and unbreakable. The handling thus becomes easier. It is non-corrosive, hence pickles, spices, etc. can be stored in plastic containers. It is cheaper as compared to the metal and glass articles. Thus, it becomes popular with general public. It is easier to wash and maintain the plastic articles. These are some of the reasons of making plastic a domestically useful material.
Collect information:
Question 1.
To prevent the degradation due to sunlight, some materials are stored in which type of bottles?
Answer:

The amber glass or brown coloured glass bottles are used to store the materials that may get degraded due to sunlight. This colour prevents the ultraviolet rays of the sun to enter the bottle and bring about chemical changes in the materials. Especially, for sensitive medicines, brown glass bottles are used.
![]()
Question 2.
Which type of glass is used in vehicles to avoid injuries in accidents?
Answer:
Front and rear doors have tempered glass. The windshield has laminated safety glass. Even if the accident takes place the glass pieces do not injure the passengers. Laminated safety glass combines two curved glass sheets and a plastic lamina between the two layers of the glass.
This glass is tough and protective. Tempered glass on the other hand is toughened glass which is processed by controlled thermal or chemical treatments. Upon breaking, the tempered glasses crumble into small granular chunks instead of splintering into sharp glass pieces. This prevents the injuries.
Sketch and label the diagram of glass manufacturing process:
Open-Ended Questions:
Question 1.
Classify and make a chart of the materials used in various items in house. Make additions to that chart with reference to various materials.
Answer:
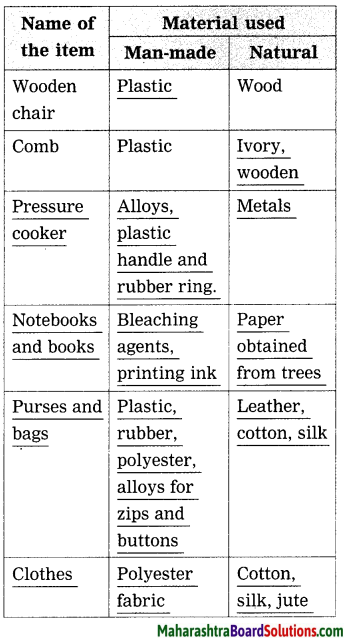
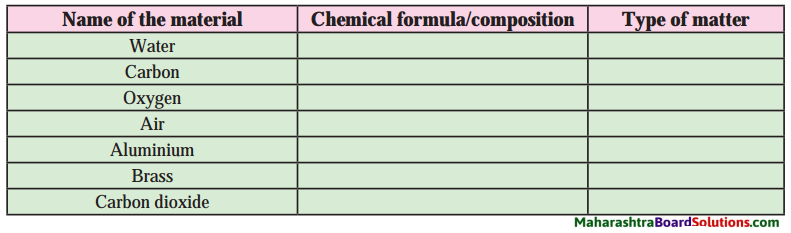
Question 2.
Make list about use of thermocol in your daily life.
Answer:
- Decorative items used at the time of festivals.
- Packing materials.
- Insulating boxes to keep food warm.
- Insulating boxes to keep fish in iced condition to prevent decomposition.
- Thermocol beads in the bean bags.