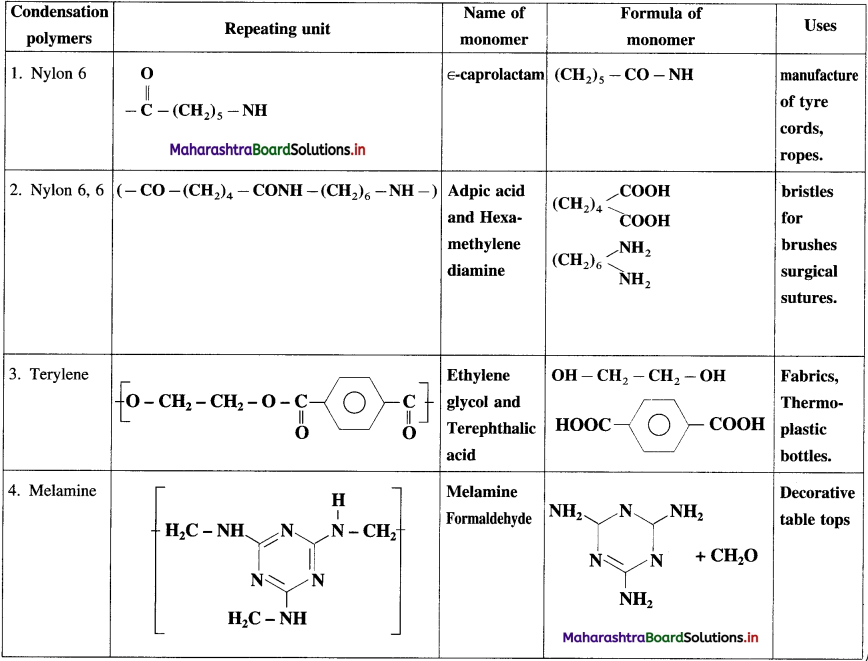
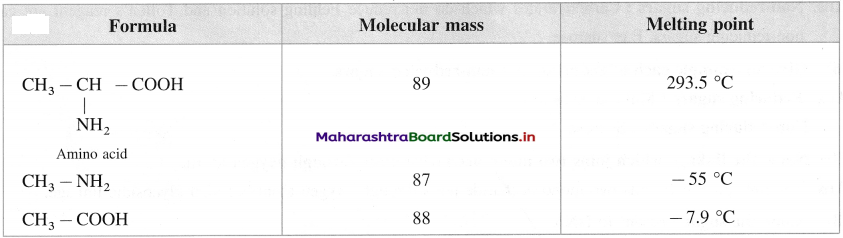
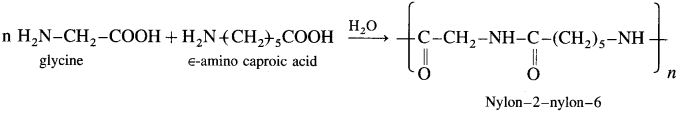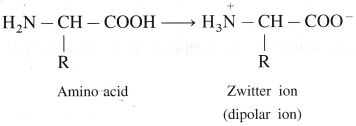Balbharti Maharashtra State Board 12th Chemistry Important Questions Chapter 8 Transition and Inner Transition Elements Important Questions and Answers.
Maharashtra State Board 12th Chemistry Important Questions Chapter 8 Transition and Inner Transition Elements
Question 1.
What are d-block elements? Give their general electronic configuration.
Answer:
Definition: d-block elements are defined as the elements in which the differentiating electron enters d-orbital of the penultimate shell i.e. (n – 1) d-orbital where ‘n is the last shell.
The general electronic configuration can be represented as, (n – n) dn – 10, nsn – 2
![]()
Question 2.
What is the position of the transition elements in the periodic table?
Answer:
The transition elements are placed in periods 4 to 7 and groups 3 to 12 of the periodic table.
Question 3.
In which block of the modern periodic table are the transition elements placed?
Answer:
Transition elements are placed in d-block of the modern periodic table.
Question 4.
Why are most of the d-block elements called transition elements?
Answer:
- d-block elements have electronic configuration,(n – n) dn – 10, nsl – 2. They are all metals.
- In the periodic table, they are placed between the ,s-block and p-block elements, i.e., in the groups between 2 and 13.
- They show characteristic properties which are intermediate between those of the elements of s-block and p-block.
- Hence, they show a transition in the properties from those of the most electropositive .v-block elements and less
- electropositive (or electronegative) p-block elements.
- Therefore, most of the d-block elements are called transition elements.
Question 5.
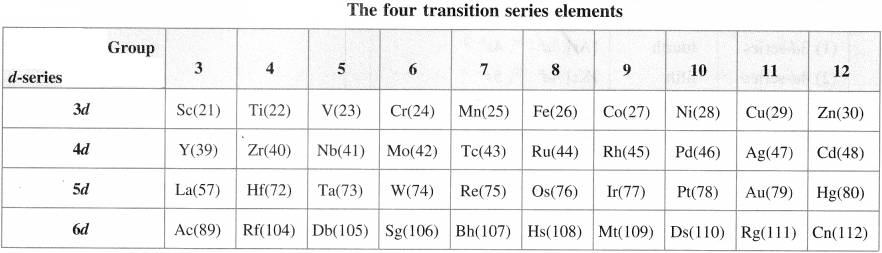
How many series of d-block elements are present in the long-form periodic table? Give their general electronic configuration.
Answer:
There are four series of d-block elements which are placed between 5 and p-block elements in the long-form periodic table as follows :
| d-series | Period | Electronic configuration |
| (1) 3d-series | fourth | [Ar] 3d1 – 10, 4s1 – 2 |
| (2) 4d-series | fifth | [Kr] 4d1 – 10, 5s1 – 2 |
| (3) 5d-series | sixth | [Xe] 4f14 5d1 – 10 6s1 – 2 |
| (4) 6d- series | seventh | [Rn] 5f14 6d1 – 10 7s2 |
Modern periodic table :

![]()
Question 6.
Represent the elements in the four series of transition elements.
Answer:
Question 7.
In which period of the periodic table, will an element, be found whose differentiating electron is a 4d-electron?
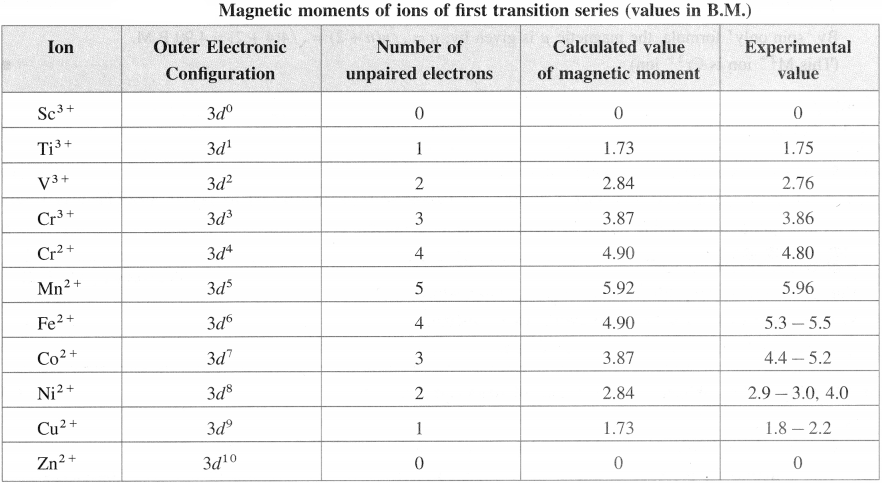
Answer:
An element whose differentiating electron is a 4d-electron will be present in fifth period of the periodic table.
Question 8.
Write the condensed electronic configuration of each series of transition elements.
Answer:
Condensed Electronic Configuration of Transition Elements


![]()
Question 9.
Write expected and observed electronic configuration of 3d-series block elements.
Answer:
Electronic configuration of 3d-series of d-block elements

Question 10.
Explain why transition elements with electronic configuration 3d44s2 and 3d94s2 do not exist.
Answer:
(1) d-orbitals are degenerate orbitals and they acquire extra stability when half-filled (3d5) or completely filled (3d10). Hence 3d4 and 3d9 electronic configurations are less stable.
(2) The energy difference between 3d and 4.s’ subshells is very low, hence there arises a transfer of one electron from 45 orbital to 3d orbital.
The electronic configuration changes as,
3d4, 4s2 → 3d5 4s1
3d9, 4s2 → 3d10 451
Therefore transition elements, with electronic configurations 3d4, 4s2 and 3d9, 4s2 do not exist.
Question 11.
Write observed electronic configuration of elements from first transition series having half-filled d-orbitals.
Answer:
There are two elements namely Cr and Mn which have half-filled d-orbitals.
24Crls22s22p63s23p63d54s1
25Mnls22s22p63s23p63d54s2
Question 12.
Explain the variable oxidation states of metals of first transition series.
Answer:
- The transition metals (or, elements) exhibit variable oxidation states due to their electronic configuration, (n – 1) d1 – 10 ns1 – 2 for the first row.
- They show only positive oxidation states due to loss of electrons from outer 45-orbital and the penultimate 3rf-orbital.
- Loss of one 45 electron forms M+ ion. Loss of two 45 electrons form M2+ ion.
- +2 is the common oxidation state of these elements.
- Higher oxidation states are due to loss of 3 d-electrons along with 45 electrons.
- As the number of unpaired electrons increases, the number of oxidation states shown by the element also increases.
- Sc has only one unpaired electron and it shows two oxidation states ( + 2 and + 3)
- Mn with 5 unpaired d electrons show six different oxidation states. They are +2, +3, +4, +5, +6 and + 7. Thus Mn has the highest oxidation state.
- From Fe onwards variable oxidation states decreases as the number of unpaired electron decreases.
- The last element in the series, Zn shows only one oxidation state ( + 2).
Question 13.
Show different oxidation states of 3d-series of transition elements.
Answer:
The following table shows, different oxidation states of 3d-series of transition elements.
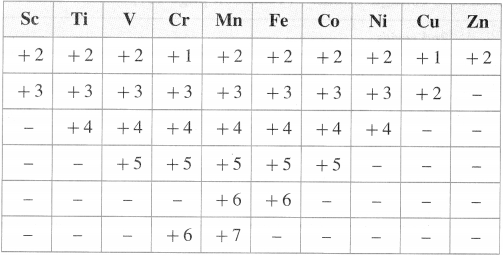
![]()
Question 14.
Write oxidation states and outer electronic configuration of first transition series elements.
Answer:
Oxidation states of first transition series elements

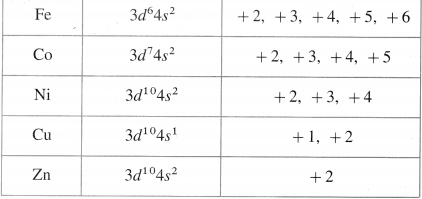
Question 15.
Zinc shows only one oxidation slate. Explain.
Answer:
- The electronic conliguration of zinc is, 30Zn Is2 2s2 2p6 3s2 3p6 3d10 4s2 or [Ar] 3d10 4s2.
- Due lo loss of two electrons from 4s suhshell Zn shows oxidation state +2. with elcctronic configuration. [Ar] 183d10.
- Since Zn+2 acquires an extra stability of completely fIlled 3d10 orbital. it shows only one oxidation state + 2.
Question 16.
Why is manganese more stable in the + 2 state than the + 3 state and the reverse is true for iron?
Answer:
- The electronic configuration of Mn is 25Mn [Ar] 3d5 4s2
- In + 2 and + 3 oxidation states, the electronic configuration of Mn is, Mn2+ [Ar] 3d5 and Mn3+ [Ar] 3d4
- Since half-filled d-orbital (3d5) has more stability and lower energy than 3d4, Mn2+ is more stable than Mn3+.
- The electronic configuration of Fe is 26Fe [Ar] 3d6 4s2 In + 2 and + 3 oxidation states of Fe, the electronic configuration is, Fe2+ [Ar] 3d6 and Fe3+ [Ar] 3d5 Since half-filled orbital is more stable, + 3 state of Fe is more stable than + 2 state.
Question 17.
What are the electronic configurations of various ions of 3d-elements?
Answer:
Electronic configuration of various ions of 3d elements
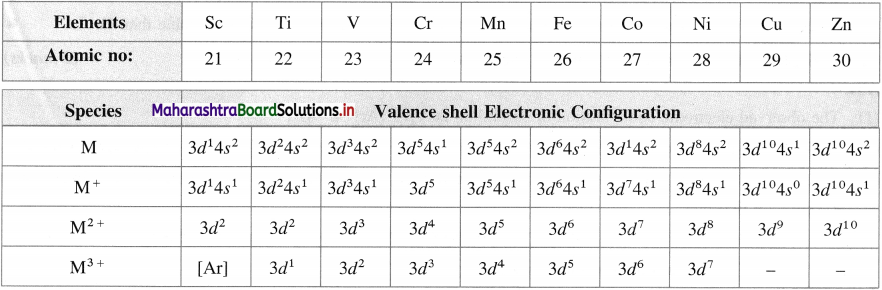
Question 18.
Scandium shows only two oxidation states. Explain.
Answer:
Scandium has electronic configuration, 21Sc : Is2, 2s2, 2p6, 3s2, 3p6, 3d1, 4s2 Sc shows only two oxidation states namely + 2 and + 3.
- Due to the loss of two electrons from the 4s-orbital, Sc acquires + 2 oxidation state Sc2 + : Is2 2s2 2p6 3s2 3p6 3d1.
- Due to the loss of one more electron from the 3d-orbital, it acquires + 3 oxidation state with the extra stability of an inert element 18Ar. Sc+3 : Is2 2s2 2p6 3s2 3p6.
- Since Sc3+ acquires extra stability of inert element [Ar]18, it does not form higher oxidation state.
![]()
Question 19.
Write different oxidation states of iron.
OR
Write the electronic configurations of
(i) Fe
(ii) Fe2+ and
(iii) Fe3+.
Answer:
Oxidation states of iron are +2, +3, +4, +5, +6.
(i) 26Fe : ls22s22p63s23p63d64s2
(ii) Fe2+ : Is2 2s2 2p6 3s2 3p6 3d6
(iii) Fe3+ : Is2 2s2 2p6 3s2 3p6 3d5.
Question 20.
Explain different oxidation states of chromium.
Answer:
- The observed electronic configuration of chromium is, 24Cr [Ar] 3d5 4s1.
- Different possible oxidation states of Cr are 4-1 (3d5), + 2 (3d4), + 3 (3d3), + 4 (3d2), + 5 (3d1) and + 6 (3d°).
- Although in + 1 state, Cr gets extra stability of half-filled 3d5-orbital, it does not exhibit + 1 state in common except with pyridine.
- Cr+2 has few stable salts like CrCl2, CrSO4 while Cr+3 forms very stable salts like CrCl3.
- Cr+4 and Cr + 5 are unstable oxidation states.
- Cr+6 is the most stable state due to inert gas [Ar] electronic configuration and form the salts like K2Cr2O7.
Question 21.
Manganese shows variable oxidation states. Give reasons.
Answer:
- Manganese (25Mn) has electronic configuration. 25Mn [Ar]18 3d5 4s2.
- Mn has stable half-filled d-subshell.
- Due to a small difference in energy between 3d and 4s-orbitals, Mn can lose or share electrons from both the orbitals, hence shows variable oxidation states.
- Mn shows oxidation states ranging from + 2 to + 7.
Question 22.
Write the different oxidation states of manganese. Why is + 2 oxidation state of manganese more stable than Mn3+?
Answer:
- The different oxidation states of Mn are +2, +3, +4, +5, + 6 and +7.
- The electronic configuration of Mn is Is2 2s2 2p6 3s2 3p6 3d5 4s2
- + 2 oxidation state is very stable due to higher stability of half-filled 3d orbital.
- Mn3+ has electronic configuration, ls22s2 2p63s23p63dA which is less stable.
Question 23.
Write the physical properties of first transition series.
Answer:
Physical properties of first transition series :
- All transition elements of the first series are metals.
- Except Zn, they are very hard and have low volatility.
- They show characteristic properties of metals. They are lustrous, malleable and ductile.
- They are good conductors of heat and electricity.
- They have high melting points and boiling points.
- Except Zn and Mn, they have one or more typical metallic structures at normal temperatures.
Question 24.
Which elements in the transition elements, 3d-series has
(i) the lowest density
(ii) the highest density?
Answer:
In 3d transition elements,
(i) Scandium (Sc) has lowest density and
(ii) Zinc (Zn) has the highest density.
![]()
Question 25.
Explain the variation in density of d-block elements.
Answer:
The densities of d-block elements are higher than 5-block elements due to higher nuclear charge which results in reduction in atomic size.
Question 26.
Explain the variation in melting points of the transition elements.
Answer:
- All transition elements are metals and the strength of metallic bonding increases as the number of unpaired electrons increases.
- In transition elements as atomic number increases, the number of unpaired electrons increases from (n – 1)d1 to (n – 1 )d5.
For example in 3d-series, melting points increase from 21Sc to 24Cr in 4d-series from 39Y to 42Mo, and in 5d-series from 72Hf to 74W. - After (n – l)d5 electronic configuration, the electrons start pairing, hence the number of unpaired electrons decrease, hence metallic character, melting points decrease from (n-1 )d6 to (n – 1)d10.
- In all transition series the melting point increases steadily up to d5 configuration and after this melting point decreases regularly.

Question 27.
The first ionisation enthalpies of third transition series elements are much higher than those of the elements of first and second transition series. Explain.
Answer:
- Third transition series elements have electronic configuration, 4f14 5d1 – 10 6s2.
- Thus, atoms of third series elements possess filled 4f-orbitals.
- 4f-orbitals due to their diffused shape, exhibit poor shielding effect and give rise to lanthanide contraction. Hence the valence electrons experience greater nuclear attraction and greater amount of energy is required to ionise the elements of third transition series namely (Hf to Au).
- Therefore the ionisation enthalpies of third transition series elements are much higher than those of the first and second transition series.

Question 28.
Explain the metalic character of transition metals.
Answer:
- All the transition elements are metals.
- They are hard, lustrous, malleable, ductile and they have high tensile strength.
- They have high melting points and boiling points.
- Their metallic character is due to vacant or partially filled (n – 1) d-orbitals, and they involve both metallic and covalent bonding.
- Since the strength of metallic bonds depends upon the number of unpaired electrons, it increases up to middle i.e., up to (n – 1 )d5, hence accordingly melting points and boiling points also increase.
- After (n – l)d5 configuration, the electrons start pairing, hence the metallic strength, melting points and boiling points decrease with the increase in atomic number.
![]()
Question 29.
How does metallic character vary in 3d transition elements?
Answer:
- In 3d-series elements as atomic number increases from scandium (Sc [Ar]18 3d1 4s2) the number of unpaired electrons increases up to 3d5 in chromium.
- As the number of unpaired electrons increases, the metallic character increases, hence the melting points and boiling points increase from 21Sc(3d1) to 24Cr (3d5).
- After chromium the number of unpaired electrons goes on decreasing due to the pairing of electrons, hence metallic character, melting points and boiling points decrease from 25Mn to 29Cu.
- Zinc has all electrons paired, hence it is soft, has a low melting and boiling points.
Question 30.
Which are the common arrangement of the atoms in the structure of transition metals?
Answer:
Most of the transition metals have simple hexagonal closed packed (hep), cubic closed packed (ccp) or body centred cubic (bcc) lattices.
Question 31.
Why do the compounds of transition metals exhibit magnetic properties?
Answer:
The compounds of transition metals exhibit magnetic properties due to the presence of unpaired electrons in their atoms or ions.
Question 32.
What is the cause of paramagnetism and ferromagnetism?
Answer:
Paramagnetism and ferromagnetism is due to the presence of unpaired electrons in species.
Question 33.
When does species become diamagnetic?
Answer:
When there is no unpaired electron, i.e. all electron spins are paired, the species become diamagnetic.
Question 34.
How do metals Fe, Co, Ni acquire permanent magnetic moment?
Answer:
The transition metals Fe, Co and Ni are ferromagnetic. When the magnetic field is applied, all the unpaired electrons in these metals (and their compounds) align in the direction of the applied magnetic field. Due to this the magnetic susceptibility is enhanced and these metals can be magnetised, that is, they acquire permanent magnetic properties.
Question 35.
In which oxidation state, is vanadium diamagnetic?
Answer:
- The electronic configuration of vanadium is, 23V [Ar] 3d3 4s2.
- In +5 oxidation state, the electronic configuration is, V5+ [Ar].
- Since in V5+ state, vanadium has all electrons paired, it is diamagnetic.
Question 36.
How is a magnetic moment expressed?
Answer:
The magnetic moment is expressed in Bohr magneton (B.M.). It is denoted by μ.
Question 37.
What is Bohr magneton (B.M.)?
Answer:
Bohr magneton (B.M.) is a unit of magnetic moment :
\(1 \mathrm{~B} . \mathrm{M} .=\frac{e h}{4 \pi m_{\mathrm{e}} c}\)
where, h : Planck’s constant (h = 6.626 x 10-34 Js)
e : electronic charge (1.60218 x 10-19 C)
me : mass of an electron (9.109 x 10-31 kg)
c : velocity of light. (2.998 x 108 ms-1)
![]()
Question 38.
Explain the magnetic properties of transition (or d-block) elements.
Answer:
- Most of the transition metal ions and their compounds are paramagnetic in nature due to the presence of one or more unpaired electrons in their (n – 1)d-orbitals. Hence they are attracted in the magnetic field.
- As the number of unpaired electrons increases from 1 to 5 in J-orbitals, the paramagnetic character and magnetic moment increase.
- The transition elements or their ions having all electrons paired are diamagnetic and they are repelled in the magnetic field.
- Metals like Fe, Co and Ni possess very high paramagnetism and acquire permanent magnetic moment hence they are ferromagnetic.
Question 39.
Explain the effective magnetic moment of the species.
Answer:
- The magnetic moment in the species arises due to the presence of unpaired electrons.
- The magnetic moment depends upon the sum of orbitals and spin contribution for each unpaired electron present in the species.
- In transition metal ions, the contribution of orbital magnetic moment is suppressed by the electrostatic field of other atoms, molecules or ions surrounding the metal ion in the compound.
- Hence the net or effective magnetic moment arises mainly due to spin of electrons. The effective magnetic moment μeff, of a paramagnetic substance is given by 1 spin only’ formula represented as, \(\mu=\sqrt{n(n+2)}\) B.M. where n is the number of unpaired electrons.
Question 40.
What is the importance of magnetic moment (μ)?
Answer:
- From the measurements of the magnetic moment (μ) of the species or metal complexes of the first row of transition elements, the number of unpaired electrons can be calculated with the spin-only formula.
- As magnetic moment is directly related to the number of unpaired electrons, value of μ will vary directly with the number of unpaired electrons.
- In 2nd and 3rd transition series, orbital angular moment is significant. Hence spin-only formula for the complexes of 2nd and 3rd transition series is not useful.
Question 41.
Calculate the magnetic moment of the following species :
(1) Cr3+
(2) Co
(3) Co3+
(4) Cu2 +.
Answer:
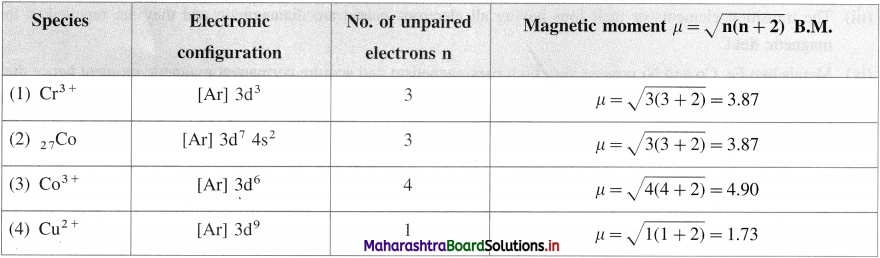
Question 42.
Explain : A slight difference in the calculated and observed values of magnetic moments.
Answer:
Magnetic moments are determined experimentally in solution or in solid state where the central atom or ion is hydrated or bound to ligands. Hence a slight difference is observed in calculated and experimentally obtained values of magnetic moment (μ).
![]()
Question 43.
Calculate the magnetic moment of a divalent ion in an aqueous solution, if its atomic number is 24.
Answer:
(1) The electronic configuration of divalent inri M2+ having atomic number 24 is.
![]()
The ion has number of unpaired electrons. n = 4.
By spin only’ formula, the magnetic μ is given by, \(\mu=\sqrt{n(n+2)}=\sqrt{4(4+2)}=4.90 \mathrm{~B} . \mathrm{M}\)
(This M2+ ion is Cr2+ ion)
Question 44.
When does a substance appear coloured?
Answer:
A substance appears coloured when it absorbs a portion of visible light. The colour depends upon the wavelength of absorption in the visible region of electromagnetic radiation.
Question 45.
Why do the d-block elements form coloured compounds?
Answer:
- Compounds (or ions) of many d-block elements or transition metals are coloured.
- This is due to the presence of one or more unpaired electrons in (n – 1) d-orbital. The transition metals have incompletely filled (n – 1) cf-orbitals.
- The energy required to promote one or more electrons within the d-orbitals involving d-d transitions is very low.
- The energy changes for d-d transitions lie in visible region of electromagnetic radiation.
- Therefore transition metal ions absorb the radiation in the visible region and appear coloured.
- Colour of ions of d-block elements depends on the number of unpaired electrons in (n – 1) d-orbital. The ions having equal number of unpaired electrons have similar colour.
- The colour of metal ions is complementary to the colour of the radiation absorbed.
Question 46.
How is complementary colour of a compound identified?
Answer:
- The transition metal ions absorb the radiation in the visible region and appeared coloured.
- Metal ion absorbs radiation of certain wavelength from the visible region. Remaining light is transmitted and the observed colour corresponds to the complementary colour of the light observed.
- The complementary colour can be identified (with the diagram given).
For example if red colour is absorbed then transmitted complementary colour is green.
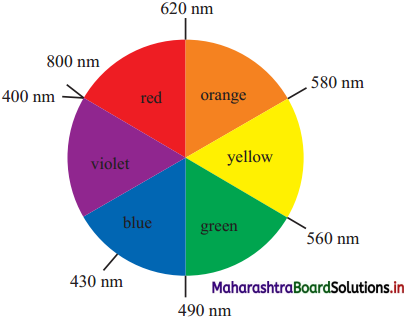
Question 47.
Write outer electronic configuration (d-orbital) and colour of 3d-series of transition metal ions.
Answer:
Colour of 3d-transition metal ions

![]()
Question 48.
Mention the factors on which the colour of a transition metal ion depends.
Answer:
The factors on which the colour of transition metal ion depends are as follows :
- The presence of incompletely filled d-orbitals in metal ions. (The compounds with the configuration d° and d’0 are colourless.)
- The presence of unpaired electrons in d-orbitals.
- d → d transitions of electrons due to absorption of radiation in the visible region.
- Nature of groups (anions) (or ligands) linked to the metal ion in the compound or a complex.
- Type of hybridisation in metal ion in the complex.
- Geometry of the complex of the metal ion.
Question 49.
Give reasons : Zinc salts are colourless.
Answer:
- Colour of the ions of d-block elements depends on the number of unpaired electrons in (n – 1) d-orbitals.
- Zinc forms salts of Zn2+ ions.
- The electronic configuration of Zn+2 is [Ar] 3d10.
- Since Zn+2 does not have unpaired electrons in 3d-orbital, d→d transition cannot take place, hence, Zn+2 ions form colourless salts.
Question 50.
Explain : The compounds of Cu(II) are coloured.
Answer:
- The electronic configuration of 29Cu [Ar] 3d10 4s1 and Cu2+ [Ar] 3d9.
- In copper compounds Cu2+ ions have incompletely filled 3d-orbital (3d9).
- Due to the presence of one unpaired electron in 3 d-orbital, Cu2+ ions absorb red light from visible spectrum and emit blue radiation due to d → d transition. Therefore, copper compounds are coloured.
Question 51.
Explain why the solution of Ti3+ salt is purple in colour.
OR
Why is Ti3+ coloured? (atomic number Ti = 22)
Answer:
- Ti2+ ions in the aqueous solution exist in the hydrated complex form as [Ti(H2O)6]2+.
- The electronic configuration of Ti is, 22Ti [Ar]18 3d2 4s2 and Ti3+ [Ar]18 3d1. Hence in complex, Ti3+ has one unpaired electron in 3d subshell.
- Initially, the 3d electron occupies lower energy d-orbital (in t2g-orbitals).
- On the absorption of radiations of about 500 nm in yellow green region by a complex, 3d1 electron is excited to the higher energy d-orbital (eg-orbitals).
- When the electron returns back to the lower energy d-orbital (t2g), it transmits radiation of complementary colour i.e. red blue or purple colour. Hence, the solution of hydrated Ti3+ is purple.
Question 52.
What will be the colour of Cd2+ salts? Explain.
Answer:
- The electronic configuration of, 48Cd [Kr]36 3d10 5s2 and Cd2+ [Kr]36 3d10.
- Cd2+ ions have completely filled 3d subshell and there are no unpaired electrons in 3d-orbital.
- Hence d → d transition is not possible.
- Therefore, Cd2+ ions do not absorb radiations in the visible region and the salts of Cd2+ ions are colourless (or white).
![]()
Question 53.
Indicate which of the ions may be coloured- V3+, Sc3+, Cr31, Cu2+, Ti3+, Cu+
Answer:
- V3+ [Ar]18 3d2-((green)
Since there are two unpaired electrons available, for d → d transition, it will show a Green colour. - Sc3+ [Ar]18 3d° (colourless/white).
Since there are no unpaired electrons in the 3d subshell, it will not show colour. - Cr3+ [Ar]18 3d3 – (violet)
There are three unpaired electrons in the 3d subshell, hence due to d → d transition, it will show violet colour. - Cu2+ [Ar]18 3d9 (blue)
It has one unpaired electron that can undergo a d → d transition, hence it will show the colour blue. - Ti3+ [Ar]18 3d1 (purple)
It has one unpaired electron that can undergo a d → d transition, hence it will show the colour purple. - Cu1+ [Ar]18 3d10 (colourless)
There are no unpaired electrons in the 3d subshell, hence it will not show colour.
Question 54.
Explain why is cobalt chloride pink in colour when dissolved in water but turns deep blue when treated with concentrated hydrochloric acid.
Answer:
- The electronic configuration of 27Co : [Ar] 3d14s2 and Co2+ [Ar] 3d1.
- When dissolved in water cobalt chloride, Co2+ forms pink complex, [Co(H2O)6]2+.
- The complex has octahedral geometry.
- Due to absorption of radiation in the visible region and d – d transition, it forms pink coloured solution.
- When CoCl2 solution is treated with concentrated HCl solution it turns deep blue.
- This change is due to the formation of another complex, [CoC14]2+ which has a tetrahedral geometry.
- Thus due to a change in geometry of the complex formed the colour of the solution changes from pink to deep blue.
Question 55.
Explain the catalytic properties of the rf-block or transition metals.
Answer:
- d-block elements or transition metals and their compounds or complexes influence the rate of a chemical reaction and hence act as catalysts.
- In homogeneous catalysis a catalyst forms an unstable intermediate compound which decomposes into products and regenerates the catalyst. But transition metals involve heterogeneous catalysis.
- The transition metals have incompletely filled d-subshells which adsorb reactants on the surface and provide a large surface area for the reactants to react.
- Since transition metals have variable oxidation states they are very good catalysts.
- Hence, compounds of Fe, Co, Ni, Pt, Pd, Cr etc are used as catalysts in many reactions.
Question 56.
Explain the use of different transition metals as catalysts.
Answer:
The transition metals are very good catalysts.
- MnO2 is used as a catalyst in the decomposition of KClO3.
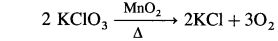
- In the manufacture of ammonia by Haber’s process, Mo/Fe is used as a catalyst.
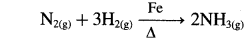
- In the synthesis of gasoline by Fischer Tropsch process, Co-Th alloy is used as a catalyst.
- Finely divided Ni (formed by reduction of heated oxide in hydrogen) is very efficient catalyst in hydrogenation of ethene to ethane at 140 °C.

- Commercially, hydrogenation with Ninkel as catalyst is used to convert inedible oils into solid fat for the production of margarine.
- In the contact process of industrial production of sulphuric acid, sulphur dioxide and oxygen (from air) react reversibly over a solid catalyst of platinised asbestos.

- Carbon dioxide and hydrogen are formed by the reaction of carbon monoxide and steam at 500 °C with Fe-Cr catalyst.

![]()
Question 57.
What are interstitial compounds of transition metals?
Answer:
- The interstitial compounds of the transition metals are those which are formed when small atoms like H, C or N are trapped inside the interstitial vacant spaces in the crystal lattices of the metals.
- Sometimes, sulphides and oxides are also trapped in the crystal lattices of transition elements.
- Presence of these elements in the crystal lattices of metals provide new properties to the metals.
Question 53.
Give one example of an interstitial compound.
Answer:
Steel and cast iron are examples of interstitial compounds of carbon and iron.
Question 54.
Give examples of interstitial compounds where the property of the transition metal is changed.
Answer:
Steel and cast iron are interstitial compounds of carbon and iron (carbides of iron). Due to the presence of carbon, the malleability and ductility of iron is reduced while its tenacity increases.
Question 55.
What are the properties of the interstitial compounds of transition metals?
Answer:
- The chemical properties of the interstitial compounds are the same as that of parent transition metals.
- They are hard and show the metallic properties like electrical and thermal conductivity, lustre, etc.
- Since metal-non-metal bonds in the interstitial compounds are stronger than metal-metal bonds in pure metals, the compounds have very high melting points, higher than the pure metals.
- They have lower densities than the parent metal.
- The interstitial compounds containing hydrogen (i.e., hydrides of metals) are powerful reducing agents.
- The compounds containing carbon, hence behaving as carbides, are chemically inert and extremely hard like diamond.
- In these compounds, malleability and ductility are changed. For example steel and cast iron.
Question 56.
What are interstitial compounds? Why do these compounds have higher melting points than corresponding pure metals?
Answer:
- The interstitial compounds of the transition metals are those which are formed when small atoms like H, C or N are trapped inside the interstitial vacant spaces in the crystal lattice of the metals.
- Since metal-nonmetal bonds in the interstitial compounds are stronger than metal-metal bonds in pure metals, the compounds have very high melting points, higher than the pure metals.
Question 57.
Explain the formation of alloys of transition metals.
Answer:
- The transition metals form a large number of alloys among themselves, which are hard with high melting points.
- During alloy formation atoms of one metal are distributed randomly in the lattice of another metal.
- The metals with similar atomic radii and similar properties readily form alloys.
- These alloys have industrial importance.
- The alloys can be ferrous alloys or nonferrous alloys.
Question 58.
How are the transition metal alloys classIfied?
Answer:
The transition metal alloys are classified into
- Ferrous alloys
- Nonferrous alloys.
Question 59.
Explain what are
(1) ferrous alloys and
(2) nonferrous alloys.
Answer:
- Ferrous alloys: In ferrous alloys, atoms of other elemems are distributed randomly in atoms of iron in the mixture. As the percentage of iron is more in these alloys, they are termed as ferrous alloys. For expamle : nickel steel, chromium steel, stainless steel, (All steels have abot 2% carbon)
- onferrous alloys : These are formed by mixing atoms of transition metal other than iron with a non transition elemeni. For example, brass is an alloy of Cu and Zn. Bronze is an alloy of Cu and Sn.
![]()
Question 60.
What are the uses of alloys?
Answer:
| Name of alloy | Important use in industry |
| (1) Bronze (Cu + Sn) | In making statues, medals and trophies (as it is tough, strong and corrosion-resistant) |
| (2) Cupra-nickel (Cu + Ni) | In making machinery parts of marine ships, boats, marine condenser tubes. |
| (3) Stainless steel | In the construction of the outer fuselage of ultra-high-speed aircraft. |
| (4) Nichrome : (Ni+ Cr in the ration 80 : 20) | For gas turbine engines. |
| (5) Titanium alloys | For ultra-high-speed flight, fireproof bulkheads and exhaust shrouds (as they withstand high temperatures). |
Question 61.
Write the preparation of potassium permanganate.
Answer:
Potassium permanganate (KMnO4) is prepared in the following steps,
(1) Chemical Oxidation : When finely divided manganese dioxide (Mn02) is heated strongly with fused caustic potash (KOH) and an oxidising agent potassium chlorate (KCIO3), dark green potassium manganate (K2MnO4) is obtained. (In neutral or acidic medium K2MnO4 disproportionates.)
![]()
The liquid is filtered through glass wool or sintered glass and evaporated. Potassium manganate crystallises as small, blackish crystals.
(2) Oxidation of K2MnO4 by
(i) Electrolytic oxidation : An alkaline solution of manganate ion is electrolysed between iron electrodes separated by a diaphragm. Manganate ion \(\left(\mathrm{MnO}_{4}^{2-}\right)\) undergoes oxidation at anode forming permanganate ion \(\left(\mathrm{MnO}_{4}^{-}\right)\). Oxygen evolved at anode converts \(\left(\mathrm{MnO}_{4}^{2-}\right)\) to \(\left(\mathrm{MnO}_{4}^{-}\right)\).
The overall reaction is as follows :
2K2MnO4 + H2O + [O] → 2KMnO4 + 2KOH
The electrolytic solution is filtered and evaporated to obtain deep purple black crystals of KMn04.
(ii) By passing CO2 through the solution of K2MnO4 :
3K2MnO4 + 4CO2 + 2H2O → 2KMnO4 + MnO2 + 4 KHCO3
Question 62.
What is meant by the disproportionation of an oxidation state? Explain giving example of manganese.
Answer:
- Disproportionation reaction is a chemical reaction in which atom or an ion of an element forms two or more species having different oxidation states, one lower and one higher.
- Manganese (Mn) shows different oxidation states + 2 to +7.
- When one oxidation state, lower or higher oxidation state becomes unstable as compared to another oxidation state, it undergoes disproportionation reaction.
- For example, + 6 oxidation state of Mn is less stable than + 7 and + 4.
- Hence, in acidic medium \(\mathrm{Mn}^{6+} \text { in } \mathrm{MnO}_{4}^{2-}\) undergoes disproportionation reaction.

- In neutral medium green K2MnO4 disproportionates to KMn04 and MnO2.

- Hence, in acidic medium \(\mathrm{Mn}^{6+} \text { in } \mathrm{MnO}_{4}^{2-}\) undergoes disproportionation reaction.
![]()
Question 63.
Give examples of oxidising reactions of KMnO4.
Answer:
(1) KMnO4 in acidic medium :
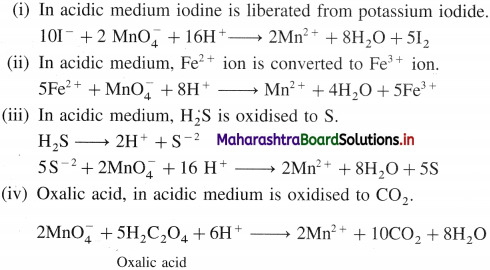
(2) KMnO4 in neutral or alkaline medium in neutral or weakly alkaline medium :
(i) Iodide is oxidised to iodate ion.
![]()
(ii) Thiosulphate ion is oxidised to sulphate ion.

(iii) Manganous salt is oxidised to MnO2.
![]()
Question 64.
Balance the following equations :
KI + KMnO4 + H2SO4 → K2SO4 + MnSO4 + 8H2O + I2
H2S + KMnO4 + H2SO4 → K2SO4 + MnSO4 + H2O + S.
Answer:
10 KI + 2KMnO4 + 8H2SO4 → 6K2SO4 + 2MnSO4 + 8H2O + 5I2
5H2S + 2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 8H2O + 5S.
Question 65.
Give the uses of potassium permanganate.
Answer:
Uses of potassium permanganate :
- as an antiseptic.
- as a powerful oxidising agent in laboratory and industry.
- in the detection of unsaturation in organic compounds in the laboratory. (Baeyer’s reagent, alkaline KMnO4).
- for detecting halides in qualitative analysis.
- in volumetric analysis for the estimation of H2O2, FeSO4 etc.)
Question 66.
Write the formula of chromite ore.
Answer:
FeOCr2O3.
Question 67.
How is potassium dichromate manufactured from chromite ore (FeOCr2O3)?
Answer:
Manufacture of potassium dichromate (K2Cr2O2) from chrome iron ore (FeOCr2O3) involves following steps :
(1) Concentration of ore : The chromite ore (FeOCr2O3) is powdered and washed with current of water.
(2) Conversion of chromite ore into sodium chromate : The concentrated ore is mixed with anhydrous sodium carbonate (Na2CO3) and a flux of lime in excess air and heated in a reverberatory furnace.
![]()
Sodium chromate (Na2CrO4) formed in the reaction is then extracted with water so that Na2CrO4 dissolves into solution and insoluble substances separate out.
(3) Conversion of Na2CrO4 into sodium dichromate (Na2Cr4O7) : Na2CrO4 solution is acidified with concentrated H2SO2, so that sodium chromate is converted into sodium dichromate.
![]()
Less soluble sodium sulphate crystallises out as Na2SO4.10H2O. which is filtered off.
(4) Conversion of Na2Cr2O7 into K2Cr2O7 : Concentrated solution of Na2Cr2O7 is treated with KCl on by double decomposition, K2Cr2O7 is obtained.
Na2Cr2O7 + 2KCl → K2Cr2O7 + 2NaCl
On concentrating and cooling the solution, less soluble orange coloured K2Cr2O7 crystallises out which is filtered and purified by recrystallisation.
![]()
Question 68.
What happens when hydrogen sulphide gas (H2S) is passed through acidified K2Cr2O7 solution?
Answer:
When hydrogen sulphide (H2S) gas is passed into solution of K2Cr2O7, H2S is oxidised to a pale yellow solid (precipitate) of sulphur. Orange coloured solution becomes green due to formation of chromic sulphate (green coloured).
In the reaction, H2S is oxidised to S and K2Cr2O7 is reduced to Cr2(SO4)3.
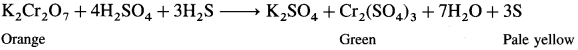
Question 69.
What are the common physical properties of d-block elements?
Answer:
The common physical properties of d-block elements are :
- All d-block elements are lustrous and shining.
- They are hard and have high density
- They have high melting and boiling points.
- They are good electrical and thermal conductors.
- They have high tensile strength and malleability.
- They can form alloys with transition and nontransition elements
- Many metals and their compounds are paramagnetic.
- Most of the metals are efficient catalysts.
Question 70.
What are the common chemical properties of d-block elements?
Answer:
The common chemical properties of the d-block elements are :
- All d-block elements are electropositive metals.
- They exhibit variable oxidation states and form coloured salts and complexes.
- They are good reducing agents.
- They form insoluble oxides and hydroxides.
- Iron, cobalt, copper, molybdenum and zinc are biologically important metals.
- They catalyse biological reactions.
Question 71.
Give examples to show that elements of first row of d-block elements differ from second and third row with respect to the stabilisation of higher oxidation states.
Answer:
- Highest oxidation state for the first row element is + 7 as in Mn.
For the second row, the highest oxidation state is + 8 as in Ru (RuO4).
For the third row, the highest oxidation state is + 8 as in Os (OsO4). - Compounds of Mo(V) of 2nd row and W(VI) of 3rd row of transitional elements are more stable than Cr(VI) and Mn (VIII) of first row elements.
Question 72.
How do metals occur in nature?
Answer:
In nature, few metals occur in earth’s crust in free state or native state while other metals occur in the combined form.
(1) Elements in free state or native state : The metals which are non-reactive with air, water, CO2 and non-metals occur in free state or native state. For example, gold, platinum, palladium occur in free state. Metals like Cu, Ag and Hg occur partly in the free state.
(2) Combined form : The metals which are reactive occur in the combined state with other elements forming compounds like oxides, sulphides, sulphates, carbonates, silicates, etc.
Question 73.
What are minerals?
Answer:
Minerals : They are naturally occurring chemical substances in the earth’s crust containing metal in free state or in combined form and obtainable from mining are called minerals. For example, haematite Fe203, galena PbS, etc.
Question 74.
What are ores?
Answer:
Ores : The minerals containing a high percentage of metals from which metals can be profitably extracted are called ores.
[Note : Every ore is a mineral but every mineral is not an ore.]
Question 75.
Write names of minerals and ores of Iron, Copper and Zinc.
Answer:
| Metals | Mineral | Ore |
| Iron | Haematite Fe2O3 Magnetite Fe3O4 Limonite 2Fe2O3, 3H2O Iron pyrites FeS2 Siderite FeCO3 | Haematite |
| Copper | Chalcopyrite CuFeS2 Chalcocite Cuprite Cu2O | Chalcopyrite Chalcocite |
| Zinc | Zinc blende ZnS Zincite ZnO Calamine ZnCO3 | Zinc blende |
![]()
Question 76.
What is metallurgy?
Answer:
Metallurgy : The process of extraction of metal in a pure state from its ore is called metallurgy.
Question 77.
Define the following:
(1) Pyrometallurgy
(2) Hydrometallurgy
(3) Electrometallurgy.
Answer:
- Pyrometallurgy : It is a process of extraction of metal from metal oxide from concentrated ore by reduction with a suitable reducing agent like carbon, hydrogen, aluminium, etc. at high temperature.
- Hydrometallurgy : It is a process of extraction of metals by converting their ores into aqueous solutions of metal compounds and reducing them by suitable reducing agents.
- Electrometallurgy : It is a process of extraction of highly electropositive metals like Na, K, Al, etc. by electrolysis of fused compounds of the metals where metal ions are reduced at cathode forming metals.
Question 78.
What is gangue?
Answer:
Gangue : The earthly and undesired impurities of various substances like sand (SiO2), metal oxides, etc. present in the ore are called gangue or matrix.
Question 79.
Define concentration of an ore.
Answer:
Concentration : A process of removal of gangue or unwanted impurities from the ore is called concentration of an ore. It is also called benefaction or dressing of an ore.
Question 80.
What are common methods of concentration of an ore?
Answer:
The concentration of an ore involves different methods depending upon the differences in physical properties of compounds or the metal present and the nature of the gangue.
The common methods of concentration of ore are as follows :
- Gravity separation or hydraulic washing :
This can be carried out by two processes as follows :- Hydraulic washing by using Wilfley’s table method
- Hydraulic classifier methods.
- Magnetic separation
- Froth floatation process.
- Leaching.
The method depends upon the nature of ore.
Question 81.
What is leaching?
Answer:
Leaching : ft is a (chemical) process used in the concentration of an ore by extracting soluble material from an insoluble solid by dissolving in a suitable solvent. This method is used in the concentration process of ores of Al, Ag, Au, etc.
Question 82.
What is roasting of an ore?
Answer:
Roasting : It is a process of strongly heating a concentrated ore in the excess of air below melting point of metal, to convert it into oxide form. It is used for a sulphide ore. For example, ZnS ore on roasting forms ZnO.
Question 83.
Write an equation to show how zinc blende (ZnS) is converted to ZnO.
Answer:
When zinc blende is roasted, it is converted to ZnO.
\(\mathrm{ZnS}+\mathrm{O}_{2} \stackrel{\Delta}{\longrightarrow} \mathrm{ZnO}+\mathrm{SO}_{2}\)
![]()
Question 84.
Explain the term : Smelting
Answer:
Smelting : The process of extraction of a metal from its ore by heating and melting at high temperature is called smelting. Reduction of ore is carried out during smelting.
Question 85.
What is calcination?
Answer:
Calcination is a process in which the ore is heated to a high temperature below the melting point of the metal in the absence of air or limited supply of air in a reverberatory furnace.
It is generally used for carbonate and hydrated oxides to convert them into anhydrous oxides.
Question 86.
Define the terms :
(1) Flux
(2) Slag
Answer:
(1) Flux : A flux is a chemical substance which is added to the concentrated ore during smelting in order to remove the gangue or impurities by chemical reaction forming a fusible mass called slag.
(2) Slag : It is a waste product formed by combination of a flux and gangue (or impurities) during the extraction of metals by smelting process.
Iron is the fourth most abundant element in the earth’s crust.
Question 87.
What is the composition of haematite ore?
Answer:
Composition of Haematite ore is Fe2O3 + SiO2 + Al2O3 + phosphates
Question 88.
Which impurities (gangue) are present in haematite ore?
Answer:
SiO2 and Al2O3 are the impurities present in the haematite ore.
Question 89.
Which reducing agents are used to reduce haematite ore into metallic iron?
Answer:
Haematite ore is reduced using coke and CO. Carbon in the coke is converted to carbon monoxide. Carbon and carbon monoxide together reduce Fe203 to metallic iron.
Fe2O3 + 3C → 2Fe + 3CO.
Fe2O3 + 3CO → 2Fe + 3CO2.
Question 90.
Why is limestone used in the extraction of iron?
Answer:
- The ore of iron contains acidic gangue or impurity of silica, SiO2.
- To remove silica gangue, basic flux like calcium oxide CaO, is required, which is obtained from the decomposition of limestone, CaCO3. \(\mathrm{CaCO}_{3} \stackrel{\Delta}{\longrightarrow} \mathrm{CaO}+\mathrm{CO}_{2}\)
- Silica reacts with CaO and forms a fusible slag of CaSiO3.
\(\mathrm{SiO}_{2}+\mathrm{CaO} \stackrel{\Delta}{\longrightarrow} \mathrm{CaSiO}_{3}\)
Therefore in the extraction of iron, lime is used.
![]()
Question 91.
Name the furnace in which iron is extracted from Haematite ore.
Answer:
Extraction of iron is carried out in Blast furnace.
Question 92.
Explain the extraction of iron from haematite.
Answer:
Iron is mainly extracted from haematite, Fe2O3 by reduction process.
Haematite ore contains silica (SiO2), alumina (Al2O3) and phosphates as impurity or gangue.
Coke is used for the reduction of ore.
To remove acidic gangue SiO2, a basic flux CaO is used which is obtained from lime stone CaCO3.
The extraction process involves following steps :
(1) Concentration of an ore : The powdered ore is concentrated by gravity separation process by washing it in a current of water. The lighter impurities (gangue) are carried away leaving behind the ore.
(2) Roasting : The concentrated ore is heated strongly in a limited current of air. During this, moisture is removed and the impurities like S, As and phosphorus are oxidised to gaseous oxides which escape.

After roasting, the ore is sintered to form small lumps.
(3) Reduction (or smelting) : The roasted or calcined ore is then reduced by heating in a blast furnace.

The blast furnace is a tall cylindrical steel tower about 25 m in height and has a diameter about 5-10m lined with fire bricks inside.
Blast furnace has three parts :
- the hearth,
- the bosh and
- the stack.
At the top, there is a cup and cone arrangement to introduce the ore and at the bottom, tapping hole for withdrawing molten iron and an outlet to remove a slag.
The roasted ore is mixed with coke and limestone in the approximate ratio of 12 : 5 : 3.
A blast of hot air at about 1000 K is blown from downwards to upwards by layers arrangement. The temperature range is from bottom 2000 K to 500 K at the top. The charge of ore from top and the air blast from bottom are sent simultaneously. There are three zones of temperature in which three main chemical reactions take place.
![]()
(i) Zone of combustion : The hot air oxidises coke to CO which is an exothermic reaction, due to which the temperature of furnace rises.
C + 1/2 O2 → CO ΔH= – 220kJ
Some part of CO dissociates to give finely divided carbon and O2.
2CO → 2C + O2
The hot gases with CO rise up in the furnace and heats the charge coming down. CO acts as a fuel as well as a reducing agent.
(ii) Zone of reduction : At about 900 °C, CO reduces Fe2O3 to spongy (or porous) iron.
Fe2O3 + 3CO → 2Fe + 3CO2
Carbon also reduces partially Fe203 to Fe.
Fe2O3 + 3C → 2Fe + 3CO
(iii) Zone of slag formation : At 1200 K limestone, CaCO3 in the charge, decomposes and forms a basic flux CaO which further reacts at 1500 K with gangue (SiO2, Al2O3) and forms a slag of CaSiO3 and Ca3AlO3.
CaCO3 + CaO + CO2.
CaO + SiO2 → CaSiO3
12CaO + 2Al2O3 → 4Ca3AlO3 + 3O2
The slag is removed from the bottom of the furnace through an outlet.
(iv) Zone of fusion : The impurities in ore like MnO2 and Ca3(PO4)2 are reduced to Mn and P while SiO2 is reduced in Si. The spongy iron moving down in the furnace melts in the fusion zone and dissolves the impurities like C, Si, Mn, phosphorus and sulphure. The molten iron collects at the bottom of furnace. The lighter slag floats on the molten iron and prevents its oxidation.
The molten iron is removed and cooled in moulds. It is called pig iron or cast iron. It contains about 4% carbon.
Question 93.
Write the reaction involved in the zone of reduction in blast furnace during extraction of iron.
Answer:
Zone of reduction : At about 900 °C, CO reduces Fe2O3 to spongy (or porous) iron.
Fe2O3 + 3CO → 2Fe + 3CO2
Carbon also reduces Fe2O3 to Fe.
Fe2O3 + 3C → 2Fe + 3CO
Question 94
Write reactions involved at different temperatures in the blast furnace.
Answer:
| Temperature K | Change taking place in the blast furnace | Reactions |
| 1. 500 K | Haematite ore loses moisture | ore xH2O → ore |
| 2. 900 K | Reduction of ore by CO | Fe2O3 + 3CO → 2Fe + 3CO |
| 3. 1200K | Limestone decomposes | CaCO3 → CaO + CO2 |
| 4. 1500K | Reduction of ore by C | Fe2O3 + 3C → 2Fe + 3CO |
| 5. 1600 K | (i) Reduction of FeO by C (ii) Fusion of iron and slag formation | FeO + C → Fe + CO CaO + SiO2 → CaSiO3 |
| 6. 2000 K | Combustion of coke | 2C + O2 → CO |
![]()
Question 95.
What is the action of carbon on Fe203 in blast furnace?
Answer:
Fe2O3 + 3C → 2Fe + 3CO
Question 96.
What is refining of metals?
Answer:
Refining of metals : The purification of impure or crude metals by removing metallic and nonmetallic impurities is known as refining of metals. H
Question 97.
How is pure iron obtained from crude iron?
Answer:
Pure iron can be obtained by electrolytic refining.
Question 98.
Name the methods of refining of metals.
Answer:
Methods of refining of metals :
- Electrorefining
- Liquefaction
- Distillation
- Oxidation m
Question 99.
What are the factors that govern the choice of extraction technique of metals?
Answer:
The choice of extraction technique is governed by the following factors.
- Nature of ore
- Availability and cost of reducing agent. (Generally, cheap coke is used).
- Availability of hydraulic power.
- Purity of metal required.
- Value of by-products. For example. SO2 obtained during the roasting of sulphide ores is important for the manufacture of H2SO4.
Question 100.
Which are the commercial forms of iron?
Answer:
Commercial forms of iron are :
- Cast iron
- wrought iron
- steel. H
Question 101.
(A) What are f-block elements?
(B) What are inner transition elements?
Answer:
(A)
- Elements in which differentiating electron enters into the pre-penultimate shell the (n – 2) f-orbital are known as f.block elements.
- They include 28 elements with atomic numbers ranging from 58-71 and atomic numbers 90 to 103 collectively.
- There are two f-series or two f-block elements, namely 4f and 5f series.
- The f-block includes two inner transition series namely the lanthanoid series. Cerium (58) to LuteUum (71) or the 4 f-block elements and the actinoid series. Thorium (90) to I.awrencium (103) or the 5f block elements.
(B) f-block elements are called inner transition elements since f-orbital lies much inside the f-orbital in relation to the transition metals, These elements have 1 to 14 electrons in their f-orbital.
Question 102.
What are fIrst inner transition elements?
Answer:
- 4f-hlock elements are called (first) inner transition elements and have partly filled inner orbitaIs or (4f) orbitais.
- They have general outer electronic configuration \((n-2) f^{1-14},(n-1) d^{0-1}, n s^{2}\).
- There are two f-series, namely 4f and 5f series, called lanthanoids and acùnoids respectively.
- They shos intermediate properties as compared to electropositive s-block elements and electronegative p-block elements. Hence they are called (first) inner transition elements.
Question 103.
What are lanthanoids (or lanthanides)?
OR
What is the lanthanoid series?
Answer:
- Lanthanoids or Lanthanoid series or Lanthanones : The series of fourteen elements from 58Ce to 71Lu in which a differentiating electron enters 4f sub-shell and follows lanthanum is called lanthanoid series and the elements are called lanthanoids.
- They have general electronic configuration, [Xe] 4f1-14 ,5d0-1, 6s2.
- They follow Lanthanum (Z = 57) in 3d-series.
![]()
Question 104.
What are rare earths?
Answer:
- Lanthanoids or 4f-block elements are called rare earths.
- Lanthanoids are never found in free state, and their minerals are not pure.
- They exhibit similar chemical properties hence cannot be extracted and separated by normal metallurgical processes.
- Lanthanoid metals are available on small scale. Therefore they are called rare earths.
Question 105.
Explain the position of lanthanoids in the periodic table.
OR
How is the position of lanthanoids justified?
Answer:
- Position of Lanthanoids in the periodic table : Group – 3; Period – 6.
- They interrupt the third transition series of t/-block elements (i.e. 5 d series) in the sixth period.
- They are 14 elements from 58Ce to 71Lu and their position is in between La and Hf. Since they follow lanthanum, they are called lanthanoids.
- They are called 4f-series elements and for the convenience, they are placed separately below the main periodic table.
- The actual position of lanthanoids is in between Lanthanum (Z = 57) and Hafnium (Z = 72).
- Their position is justified due to following reasons :
- All these elements have the same electronic configuration in ultimate and penultimate shells, one electron in 5d-orbital and two electrons in 6s-orbital.
- Group valence of all lanthanoids is 3.
- All lanthanoids from 58Ce to 71Lu have similar physical and chemical properties.
Question 106.
Explain the meaning of inner-transition series.
Answer:
A series of f-block elements having electronic configuration (n – 2)f1-14 (n – I) d0-1 ns2 placed separately in the periodic table represents inner transition series. The f-orbitals lie much inside the e/ orbitals.
Since the last electron enters pre-penultimate shell, these elements are inner transition elements.
There are two inner transition series as follows :
4f-series 58Ce → 71Lu
5f-series 90Th → 103Lr
Question 107.
Draw a skeletal diagram of the periodic table to show the position of d and/- block elements.
Answer:

![]()
Question 108.
What are the properties of lanthanoids?
Answer:
- Lanthanoids are soft metak with silvery white colour, Colour and brightness reduces on exposure to air.
- They are good conductors of heat and electricity.
- Except promethium (Pm), all are non-radioactive in nature.
- The atomic and ionic radii decrease from La to Lu. (Lanthanoid contraction).
- Coordination numbers arc greater than 6.
- They are paramagnetic.
- They become ferromagnetic at lower temperature.
- Their magnetic and optical properties are independent of environment.
- They are called rare earths as their exiractioli was difficult.
- They are abundant in earth’s crust
- All lanthanoids fonn hydroxides which are ionic and basic. l3asicity decreases with atomic number,
- They react with nitrogen to give nitrides and with halogen to give halides.
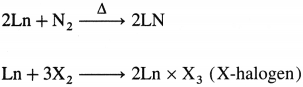
- When heated with carbon at very high temperature give carbides
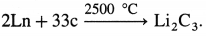
Question 109.
Explain the variations in ionisation enthalpy of lanthanoids.
Answer:
- The first ionisation enthalpy of lanthanoids is nearly same. It is very high for Gd and Yb.
- The ionisation enthalpy increases from first (IE1] to third (IE3).
First, second and third ionization enthalpies of lanthanoids in kj/mol
| Lanthanoid | IE1 | IE2 | IE3 |
| La | 538.1 | 1067 | 1850.3 |
| Ce | 528.0 | 1047 | 1949 |
| Pr | 523.0 | 1018 | 2086 |
| Nd | 530.0 | 1034 | 2130 |
| Pm | 536.0 | 1052 | 2150 |
| Sm | 543.0 | 1068 | 2260 |
| Eu | 547.0 | 1085 | 2400 |
| Gd | 592.0 | 1170 | 1990 |
| Tb | 564.0 | 1112 | 2110 |
| Dy | 572.0 | 1126 | 2200 |
| Ho | 581.0 | 1139 | 2200 |
| Er | 589.0 | 1151 | 2190 |
| Tm | 596.7 | 1163 | 2284 |
| Yb | 603.4 | 1175 | 2415 |
| Lu | 523.5 | 1340 | 2022 |
![]()
Question 110.
Give the general electronic configuration of 4f-series elements (OR lanthanoids).
Answer:
- The general electronic configuration of 4f-series elements is, Ln[Xe]54 4f1-14 5d0-1 6s2 where Ln is a lanthanoid.
- Xenon has electronic configuration, [Xe] : Is2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6.
- In lanthanoids, the differentiating electron enters prepenultimate shell, 4f m
Question 111.
What are the important features of the electronic configuration of lanthanoids?
Answer:
- Lanthanoids show two types of electronic configurations
(a) an expected or idealized
(b) an observed electronic configuration.
In the idealized electronic configuration, the filling of the 4/-orbitals is regular but in the observed configuration, there is the shift of a single electron from 5d to 4/ sub-shell. - Lanthanum (57) has an electronic configuration [Xe] 4f° 5d16s2. It does not have any f-electron.
- The next incoming electron does not enter the 5d sub-shell but goes to the 4f sub-shell.
- 14 electrons are progressively filled in the 4f sub-shell as the atomic number increases by one unit from La to Lu.
- La, Gd and Lu are the only elements which possess one electron in a 5d orbital, while in all other lanthanoids the 5d sub-shell is empty.
- La-(4f°), Gd-(4f7) and Lu-(4f14) posses extra stability due to their empty, half-filled and completely filled 4f-orbitals respectively.
- The 4f-electrons in the prepenultimate shell are shielded by the outermost higher orbitals, 5s2, 5p6, 5d1, 6s2, i.e. by eleven electrons, hence they are less effective in chemical bonding.
Electronic configuration (Idealised and observed)

[Xe]54 ls22s22p63s23p63d104s24p64d105s25p6
Question 112.
Write the expected electronic configuration of (a) Nd (Z = 60) (b) Tm (Z = 69).
Answer:
Expected electronic configuration :
(a) Nd = [Xe] 4f3 5d1 6s2
(b) Tm= [Xe] 4f145d16s2
Question 113.
Write electronic configurations of
(i) Gd
(ii) Yb.
Answer:
(i) 64Gd [Xe] 4f75d16s2 (Observed)
(ii) 70Yb [Xe] 4f145d°6s2 (Observed)
![]()
Question 114.
Write expected and observed electronic configurations of
(i) Ce
(ii) Tb.
Answer:
| Element | Expected (Idealised) | Observed |
| (i) 58Ce | [Xe] 4f15d16s2 | [Xe] 4f25d°6s2 |
| (ii) 65Tb | [Xe] 4f85d16s2 | [Xe] 4f95d°6s2 |
Question 115.
Why are the expected and observed ground state electronic configurations of gadolinium and lawrencium same?
Answer:
- The degenerate orbitals like 4f and 5f acquire extra stability when they are half filled (4f7) or completely filled (5f14).
- The expected and observed electronic configuration of gadolinium is, 64Gd [Xe] 4f7 5d1 6s2.
- The expected and observed electronic configuration of lawrencium is 103Lr [Rn] 5f14 6d1 7s2.
Question 116.
Explain oxidation states of lanthanoids.
Answer:
- The common oxidation state of the Lanthanoids is 3 + due to the loss of 2 electrons from outermost 6s orbital and one electron from the penultimate 5d sub-shell.
- Gd3+ and Lu3+ show extra stability due to their half-filled and completely filled f-orbitals, Gd3+ = [Xe]4f7, Lu3+ = [Xe]4f14
- Ce and Tb attain the 4f° and 4f7configurations in the 4 + oxidation states. Eu and Yb attain the 4f7 and 4f14 configurations in the 2 + oxidation states. Sm and Tm also show the 2+ oxidation state although their stability can be explained based on thermodynamic factors.
- Some lanthanoids show 2 + and 4 + oxidation states even though they do not have stable electronic configuration of 4f°, 4f7 or 4f14. E.g. Pr4+ (4f1), Nd2+ (4f4), Sm2+ (4f6), Dy4+ (4f8) etc
Question 117.
Write the. electronic configuration of the following ions :
(1) La3 + ;
(2) Gd3+;
(3) Eu3+;
(4) Ce3+.
Answer:
(1) La3 + = [Xe]
(2) Gd3+ = [Xe] 4f7
(3) Eu3+ = [Xe] 4f6
(4) Ce3+ = |Xe] 4f1
Question 118.
Write the electronic configuration of
(1) Nd2+
(2) Nd3+
(3) Nd4+.
Answer:
(1) Nd2+ [Xe] 4f4
(2) Nd3+ [Xe] 4f3
(3) Nd4+ [Xe] 4f2
![]()
Question 119.
Among the following lathanoids, which elements show only one oxidation state 3 +? Why? Dy, Gd, Yb, Lu.
Answer:
Gd and Lu show only one oxidation state 3 +, since they acquire electronic configurations with extra stability namely 4f7 and 4f14 respectively.
Question 120.
Write the expected electronic configurations of :
(1) europium (Z = 63),
(2) erbium (Z = 68).
Answer:
(1) Europium (63Eu) [Xe]544f6 5d1 6s2
(2) Erbium (68Er) [Xe]544f11 5d1 6s2
Question 121.
Why does lanthanum form La3+ ion, while cerium forms Ce4+ ion? (Atomic number La = 57 and Ce = 58).
Answer:
- Electronic configuration Lanthanum is La [Xe] 4f° 5d1 6s2. By losing three electrons, La acquires stable electronic configuration of Xe and forms La3+.
- Electronic configuration of Cerium is Ce [Xe] 4f1 5d1 6s2. By losing four electrons, Ce acquires stable electronic configuration of Xe and forms Ce4+.
Question 122.
63EU and 70Yb show 2 + oxidation state. Explain.
Answer:
63EU has electronic configuration, [Xe] 4f7 5d°6s2. By losing 2 electrons from 6s orbital, it acquires stable configuration and 4f-orbital is half-filled.
70Yb has electronic configuration, [Xe] 4f14 5d° 6s2. By losing 2 electrons from 6 s orbital, it acquires stable configuration and 4/-orbital is completely filled.
Hence Eu and Yb show 2 + oxidation states.
Question 123.
Display electronic configuration, atomic and ionic radii of lanthanoids.
Answer:
Answers are given in bold.
Electronic configuration and atomic ionic radii of lanthanoids

![]()
Question 124.
Explain the trend in atomic and ionic sizes of lanthanoids.
Answer:
- From 57La (187 pm) to first element of 4f-series 58Ce (183 pm), the contraction in atomic radius is very large, 4 pm.
- But from Ce onwards as atomic number increases atomic radius decreases very steadily so that total decrease in atomic radius from Ce to Lu is only 10 pm.
- In case of tripositive ions due to large pull by nucleus, the decrease in ionic radii is slightly more, i.e. 18 pm. For example, Ce3+ (103 pm) to Lu3+ (85 pm ).
- Hence all lanthanoids have similar properties. Therefore they cannot be separated from each other easily by normal metallurgical methods but require special methods.

Question 125.
What is meant by lanthanoid contraction?
Answer:
Lanthanoid contraction : The gradual decrease in atomic and ionic radii of lanthanoids with the increase in atomic number is called lanthanoid contraction.
Question 153.
Explain the causes of the lanthanoid contraction.
Answer:
The causes of the lanthanoid contraction are as follows :
- As the atomic number of lanthanoids or 4f-block elements increases the positive nuclear charge increases and correspondingly electrons are added to the prepenultimate 4f sub-shell.
- The attraction of nucleus on 4 f-electrons increases with the increase in atomic number.
- The outer eleven electrons namely, 5s2, 5p6, 5d3 and 6s2 do not shield inner 4 f-electrons from the nucleus.
- There is imperfect shielding of each 4f-electron from other 4 f-electrons.
- As compared to d sub-shell, the extent of shielding for 4 f-electrons is less.
- Due to these cumulative effects, 4 f-electrons experience greater nuclear attraction and hence valence shell is pulled towards the nucleus to the greater extent decreasing atomic and ionic radii appreciably.
- From 57La to 58Ce, there is a sudden contraction in atomic radius from 187 pm to 183 pm but the further decrease up to the last 4f-element, 71Lu is comparatively low (about 10 pm).
Question 126.
Explain lanthanoid contraction effect with respect to (1) decrease in basicity, (2) ionic radii of post-lanthanoids.
Answer:
The lanthanoid contraction has a definite effect on the properties of lanthanoids as well as on the properties of post-lanthanoid elements.
(1) Decrease in basicity :
- In lanthanoids due to lanthanoid contraction, as the atomic number increases, the size of the lanthanoid atoms and their try positive ions decreases, i.e. from La3+ to Lu3+.
- As size of the cation decreases, according to Fajan’s rule, the polarizability increases and thus the covalent character of the M-OH bond increases, and ionic character decreases.
- Therefore the basic nature of the hydroxides decreases.
- Basicity and ionic character decrease in the order La(OH)3 > Ce(OH)3 > … Lu(OH)3.
![]()
(2) Ionic radii of post-lanthanoids :
- Elements following the lanthanoids in the 6th period (third transition series, i.e. 5d-series) are known as post-lanthanoids.
- Due to lanthanoid contraction the atomic radii (size) of elements which follow lanthanum in the 6th period (3rd transition series – Hf, Ta, W, Re)-are similar to the elements of the 5th period (4d-series Zr, Nb Mo, Tc).
- Due to similarity in their size, post-lanthanoid elements (5d-series) have closely similar properties to the elements of the 2nd transition series (4d-series) which lie immediately above them.
- Pairs of elements namely Zr-Hf(Gr-4), Nb-Ta (Gr-5), Mo-W(Gr-6), Tc-Re (Gr-7) are called chemical twins since they possess almost identical sizes and similar properties.
Question 127.
Why do lanthanoids form coloured compounds?
Answer:
- The colour in lanthanoid ions is due to the presence of unpaired electrons in partially filled 4f sub-shells.
- Due to the absorption of radiations in the visible region there arises the excitations of the unpaired electrons from f-orbital of lower energy to the f-orbital of higher energy-giving f → f transitions.
- The observed colour is complementary to the colour of the light absorbed.
- The colour of try positive ions (M3+) depends upon the number of unpaired electrons in f-orbitals. Hence the lanthanoid ions having equal number of unpaired electrons have similar colour.
- The colours of M3+ ions of the first seven lanthanoids, La3+ to Eu3+ are similar to those of seven elements Lu3+ to Tb3+ in the reverse order.
Question 128.
Explain, why Ce3+ ion is colourless.
Answer:
- The electronic configuration of Ce3+ is, [Xe] 4f7
- Even though there is one unpaired electron in 4f sub-shell, the f → f transition involves very low energy. Hence, Ce3+ ion does not absorb radiation in the visible region.
Therefore Ce3+ ion is colourless.
Question 129.
Explain why Gd3+ is colourless.
Answer:
- Gd3+ has electronic configuration, [Xe] 4f7
- Due to extra stability of half filled orbital, it does not allow f → f transition, and hence does not absorb radiations in the visible region.
Hence Gd3+ is colourless.
Question 130.
The salts of (1) La3+ and (2) Lu3+ are colourless. Explain.
Answer:
(1) (i) La3+ has electronic configuration, [Xe] 4f°
(ii) Since there are no unpaired electrons in 4 f-orbital, f → f transition is not possible. Hence La3+ ions do not absorb radiations in visible region, and they are colourless.
(2) (i) LU3+ has electronic configuration [Xe] 4 f14
(ii) Since there are no unpaired electrons in 4f-orbital, f → f transition is not possible. Hence Lu3+ ions do not absorb radiations in visible region and they are colourless.
Question 131.
Explain giving examples, the colour of nf electrons is about the same as those having (14-n) electrons.
Answer:
(1) Consider Pr3+ and Tm3+ ions.
Tm3+ (4f12) has nf electron 12 electrons.
Pr2+ (4f2) has (14 – n) = (14 – 2) = 12 electrons. Both, Tm3+ and Pr3+ are green.
(2) Consider Nd3+ and Er3+ ions. Er3+ (4f11) has nf electrons 11.
Nd3+ (4f3) has (14 – n) is (14 – 3) = 11 electrons. These both ions Er3+, Na3+ are pink in colour.
Question 132.
Lu3+ has observed magnetic moment zero. How many unpaired electrons are present?
Answer:
Since magnetic moment is zero, it has no unpaired electrons.
![]()
Question 133.
What are the application of lanthanoids?
Answer:
- Lanthanoid compounds are used inside the colour television tubes and computer monitor. For example mixed oxide (Eu, Y)2 O3 releases an intense red colour when bombarded with high energy electrons.
- Lanthanoid ions are used as active ions in luminescent materials. (Optoelectronic application)
- Nd : YAG laser is the most notable application. (Nd : YAG = neodymium doped ytterium aluminium garnet)
- Erbium doped fibre amplifiers are used in optical fibre communication systems.
- Lanthanoids are used in cars, superconductors and permanent magnets.
Question 134.
What are actinoids? Give their general electronic configuration.
Answer:
- Actinoids : The series of fourteen elements from 90Th to 103Lr which follow actinium (89Ac) and in which differentiating electrons are progressively filled in 5f-orbitals in prepenultimate shell are called actinoids.
- Their general electronic configuration is, [Rn]86 5f1-14 6d0-1 7s2.
Question 135.
Why are actinoids called inner transition elements?
Answer:
- Actinoids are 5f-series elements in which electrons progressively enter into 5f-orbitals, which are inner orbitals.
- They have electronic configuration [Rn]86 5f1-14 6d0-1 7s2.
- They show intermediate properties as compared to electropositive 5-block elements and electronegative p-block elements. Hence they are called second inner transition elements.
Question 136.
Explain the position of actinoids in the periodic table.
OR
What is the position of actinoids in the periodic table?
Answer:
- Position of actinoids in the periodic table : Group-3; Period-7.
- They interrupt the fourth transition series (6d series) in the seventh period in the periodic table.
- After Actinium, 89Ac which has electronic configuration [Rn] 6d17s2, the electrons enter progressively 5f orbital and they have general electronic configuration, [Rn] 5f1 – 14 6d0 – 1 7s2.
- They are fourteen elements from 90Th to 103Lr and since they follow actinium, they are called actinoids.
- They are called 5f series or second inner transition series elements and for the convenience they are placed separately below the periodic table.
Question 137.
Write idealised and observed electronic configuration of actinoids.
Answer:
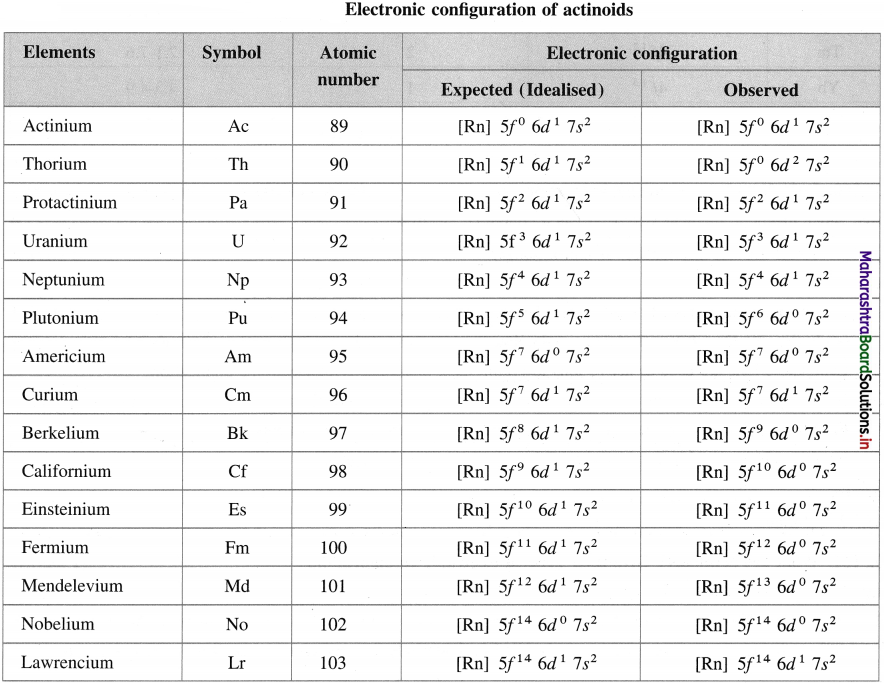
![]()
Question 138.
Explain the oxidation states of actinoids.
Answer:
- Due to availability of electrons in 5f, 6d and 7s sublevels, lanthanoids show varied oxidation states.
- The most common oxidation state is + 3 due to loss of one electron from 6d and two electrons from 6s-orbitals.
- Ac, Th and Am show + 2 oxidation state.
- Th, Pa, U, Np, Pu, Am and Cm show + 4 oxidation state.
- Np and Pu show the highest oxidation state + 7.
- U, Np, Bk, Cm and Am show stable oxidation state + 4.
- In + 6 oxidation state, due to high charge density the actinoid ions form oxygenated ions, e.g. \(\mathrm{UO}_{2}^{+}, \mathrm{NpO}_{2}^{+},\) etc.

Question 139.
Why do actinoids show variable oxidation states?
Answer:
- The large number of variable oxidation states of actinoids is due to very small energy difference between 5f, 6d and 7s subshells.
- The electronic configuration of actinoids is, [Rn] 5f1-14 6d0-1, 7s2
- Due to the loss of three electrons from 6d1 and 7s2, the common oxidation state is + 3, but due to further loss of electrons from 5f subshell, actinoids show higher oxidation states.
- The variable oxidation states are + 2 to + 7.
Electronic configuration of actinoids and their ionic radii in + 3 oxidation state
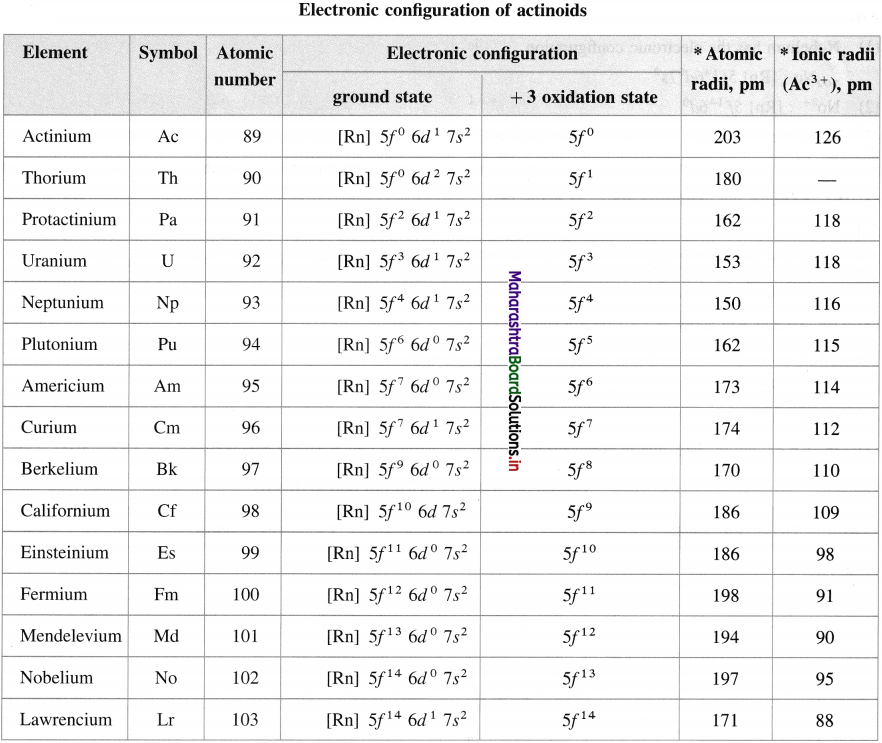
![]()
Question 140.
What is meant by actinoid contraction?
Answer:
Actinoid contraction: The gradual decrease in atomic and ionic radii of actinoids with the increase in atomic number is called actinoid contraction.
Question 141.
The extent of actinoid contraction is greater than lanthanoid contraction. Explain Why?
Answer:
- The electronic configurations of :
Lanthanoids [Xe] 4f1 – 14 5d0 – 1 6s2
Actinoids [Rn] 5f1 – 14, 6d0 – 1 7s2 - The mutual screening offered in case of 5f-electrons is less than that in the 4f-electrons.
- Hence, the outer orbitals are pulled to the greater extent by nuclei in actinoids (5f-series) than in lanthanoids (4f-series).
- Therefore, actinoid contraction is greater than lanthanoid contraction.
Question 142.
Describe the important properties of actinoids.
Answer:
Properties of actinoids :
- Actinoids are silvery white ( similar to lanthanoids).
- They are highly reactive radioactive elements.
- Most of these elements are not found in nature. They are radioactive and man made.
- They experience decrease in the atomic and ionic radii from Ac to Lw, known as actinoid contraction.
- The common oxidation state is +3. Elements of the first half of the series exhibit higher oxidation states.
Question 143.
What are the applications of actinoids?
Answer:
- Thorium oxide (ThO2) with 1% CeO2 is used as a major source of indoor lighting, as well as for outdoor camping.
- Uranium is used in the nuclear reactors.
- The isotopes of Thorium and Uranium have very long half-life, so that we get very negligible radiation from them: Hence they can be used safely.
Question 144.
What are transuranic elements?
Answer:
- The man-made elements heavier titan Uranium (Z = 92) in the Actinoid señes are called transuranic elements.
- These are synthetically or artificially prepared (man-made) elements starting from Neptunium (Z= 93).
- Transuranic elements arc generally considered to be from Neptunium (Z = 93) to Lawrencium (Z = 103).
- Recently elements from atomic number 104 (Rf) to atomic number 118 (Og) or (Uuo) in 6 d series have also been identified as transuranic elements.
- All transuranic elements are radioactive.
Question 145.
What are post actinoid elements?
Answer:
- Elements from atomic number 104 to 118 are called postactinoid elements.
- The post actinoid elements known so far are transition metals.
- They can be synthesised in the nuclear reactions.
- As they have very short half life period, it is difficult to study their chemistry.
- Ruiherfordium forms a chloride (RfCl4) similar to zirconium and hafnium in + 4 oxidation state.
- Dubniurn resembles niobium and protactinium.
![]()
Question 146.
Name the transuranic elements.
Answer:
Names of transuranic elements
| Name | Symbol | Atomic number |
| Neptunium | Np | 93 |
| Plutonium | Pu | 94 |
| Americium | Am | 95 |
| Curium | Cm | 96 |
| Berkelium | Bk | 97 |
| Californium | Cf | 98 |
| Einsteinium | Es | 99 |
| Ferminum | Fm | 100 |
| Mendelevium | Md | 101 |
| Nobelium | No | 102 |
| Lawrencium | Lr | 103 |
| Rutherfordium | Rf | 104 |
| Dubnium | Db | 105 |
| Seaborgium | Sg | 106 |
| Bohrium | Bh | 107 |
| Hassium | Hs | 108 |
| Meitnerium | Mt | 109 |
| Darmstadtium | Uun/Ds | 110 |
| Roentgenium | Uuu/Rg | 111 |
| Copernicium | Uub/Cn | 112 |
| Ununtrium | Uut | 113 |
| Ununquadium | Uuq | 114 |
| Ununpentium | Uup | 115 |
| Ununhexium | Uuh | 116 |
| Ununseptium | Uus | 117 |
| Ununoctium | Uuo | 118 |
In the transuranic elements, elements from atomic number 93 to 103 are actinoids and from atomic number 104 to 118 are called postactinoid elements.
Question 147.
What are the similarities between lanthanides and actinides.
Answer:
Lanthanides and actinides show similarities as follows :
- Both, lanthanides and actinides show+ 3 oxidation state.
- In both the series, the f-orbitals are filled gradually.
- Ionic radius of the elements in both the series decreases with increase in atomic number.
- Electronegativity in both the series is low for all the elements.
- They all are highly reactive.
- The nitrates, perchlorates and sulphates of all elements are soluble while their hydroxides, theorides and carbonates
are insoluble.
![]()
Question 148.
Differentiate between lanthanoids and actinoids.
Answer:
| Lanthanoids | Actinoids |
| Electronic configuration [Xe] 4f1-14 5d0-1, 6s2 | Electronic configuration [Rn] 5f1-14 6d0-1, 7s2 |
| The differentiating electron enters the 4f subshell. | The differentiating electron enters the 5f subshell. |
| Except for Promethium all other elements occur in nature. | Except for Uranium and Thorium, all others are synthesized in the laboratory. |
| The binding energy of 4f electrons is higher. | 5f-orbitals have lower binding energy. |
| Only Promethium is radioactive. | All elements are radioactive. |
| Besides 3 + oxidation state they show 2 + and 4 + oxidation states. | Besides 3 + oxidation state they show 2 + , 4 + , 5 + , 6 + , 7 + oxidation states. |
| They have a less tendency to form complexes. | They have greater tendency to form complexes. |
| Many lanthanoid ions are colourless. Their colour is not as deep and sharp as actinoids. | Actinoids are coloured ions. Their colour is deep, e.g. U3+ is red and U4+ is green. |
| Lanthanoids cannot form oxo-cations. | Actinoids form oxo-cations such as – UO2+, PuO2+, UO22+, PuO22+. |
| Lanthanoid hydroxides are less basic. | Actinoid hydroxides are more basic. |
| Lanthanoid contraction is relatively less. | Actinoid contraction from element to element is comparatively more. |
| Mutual shielding of 4f electrons is more. | Mutual shielding effect of 5f electrons is less. |
Question 149.
Compare Pre-transition metals, Lanthanoid and transition metals.
Answer:

![]()
Multiple Choice Questions
Question 150.
Select and write the most appropriate answer from the given alternatives for each sub-question :
1. In transition elements, the different electron enters into
(a) ns subshell
(b) np subshell
(c) (n – 1) d subshell
(d) (n – 2)f subshell
Answer:
(c) (n – 1) d subshell
2. Chromium (Z = 24) has electronic configuration
(a) [Ar]4dA 4s2
(b) [Ar] 4d5 451
(c) [Ar] 3d5 3s1
(d) [Ar] 3d5 4s1
Answer:
(d) [Ar] 3d5 4s1
3. Manganese achieves the highest oxidation state in its compounds
(a) Mn3O4
(b) KMnO4
(c) K2MnO4
(d) MnO2
Answer:
(b) KMnO4
4. The group which belongs to transition series is
(a) 2
(b) 7
(c) 13
(d) 15
Answer:
(b) 7
5. The last electron of transition element is called
(a) s-electron
(b) p-electron
(c) d-electron
(d) f-electron
Answer:
(c) d-electron
6. Which one of the following elements does NOT belong to first transition series?
(a) Fe
(b) V
(c) Ag
(d) Cu
Answer:
(c) Ag
7. The incomplete d-series is
(a) 3d
(b) 4d
(c) 5d
(d) 6d
Answer:
(d) 6d
![]()
8. The electronic configuration of Sc is
(a) [Ar] 3d2 4s2
(b) [Ar] 3d1 4s2
(c) [Kr] 3d1 4s2
(d) [Kr] 3d2 4s1
Answer:
(b) [Ar] 3d1 4s2
9. The observed electronic configuration of copper is
(a) [Ar]18 3d9 4s2
(b) [Kr] 3d10 451
(c) [Kr] 3d9 4s2
(d) [Ar] 3d10 451
Answer:
(d) [Ar] 3d10 451
10. Fe belongs to the
(a) 3d-transition series elements
(b) 4d-transition series elements
(c) 5d-transition series elements
(d) 6d-transition series elements
Answer:
(a) 3d-transition series elements
11. Which one of the following elements does not exhibit variable oxidation states?
(a) Iron
(b) Copper
(c) Zinc
(d) Manganese
Answer:
(c) Zinc
12. In KMnO4, oxidation number of Mn is
(a) 2+
(b) 4 +
(c) 6 +
(d) 7+
Answer:
(d) 7+
13. Which one of the following transition elements shows the highest oxidation state?
(a) Sc
(b) Ti
(c) Mn
(d) Zn
Answer:
(c) Mn
14. The colour of transition metal ions is due to
(a) s → s transition
(b) d → d transition
(c) p → p transition
(d) f → f transition
Answer:
(b) d → d transition
15. Which one of the following compounds is expected to be coloured?
(a) AgNO3
(b) CuSO4
(c) ZnCl2
(d) CuCl
Answer:
(b) CuSO4
![]()
16. The metal ion which is NOT coloured, is
(a) Fe3+
(b) V2+
(c) Zn2+
(d) Ti3+
Answer:
(c) Zn2+
17. A pair of coloured ion is
(a)Cu2+, Zn2+
(b)Cr3+ , Cu+
(c) Cd2+, Mn5+
(d) Fe2+, Fe3+
Answer:
(d) Fe2+, Fe3+
18. The highest oxidation state is shown by
(a) Fe
(b) Mn
(c) Os
(d) Cr
Answer:
(c) Os
19. Transition elements are good catalysts since
(a) they show variable oxidation states
(b) they have partially filled d-orbitals
(c) they have low I.P
(d) they have small atomic radii
Answer:
(a) they show variable oxidation states
20. Highest magnetic moment is shown by the ion
(a) V3+
(b) Co3+
(c) Fe3+
(d) Cr3+
Answer:
(c) Fe3+
21. The most common oxidation state of lanthanoids is
(a) +4
(b) +3
(c) +6
(d) +2
Answer:
(b) +3
22. Which one of the following elements belong to the actinoid series?
(a) Cerium
(b) Lutetium
(c) Thorium
(d) Lanthanum
Answer:
(c) Thorium
23. The total number of elements in each of f-series is
(a) 10
(b) 12
(c) 14
(d) 15
Answer:
(c) 14
![]()
24. The general electronic configuration of Lanthanoids is
(a) [Xe] 4f1 – 14 5d0 – 1 6s2
(b) [Xe] 4f2 – 14 5d0 – 1 6s2
(c) [Xe] 4f1 – 13 5d0 – 1 6s2
(d) [Xe] 4f0 – 14 5d0 – 1 6s1
Answer:
(a) [Xe] 4f1 – 14 5d0 – 1 6s2
25. f-block elements are called ………………….
(a) transition elements
(b) representative elements
(c) inner transition elements
(d) alkalin earth metals
Answer:
(c) inner transition elements
26. Actinoids form coloured salts due to the transition of electrons in
(a) d – d
(b) f – f
(c) f – d
(d) s – f
Answer:
(b) f – f
27. In the periodic table, Gadolinium belongs to
(a) 4th Group 6th period
(b) 4th group 4th period
(c) 3rd group 5th period
(d) 3rd group 7th period.
Answer:
(d) 3rd group 7th period.
![]()
28. The transuranic elements are prepared by
(a) addition reaction
(b) substitution reactions
(c) decomposition reaction
(d) nuclear reactions
Answer:
(d) nuclear reactions






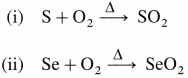
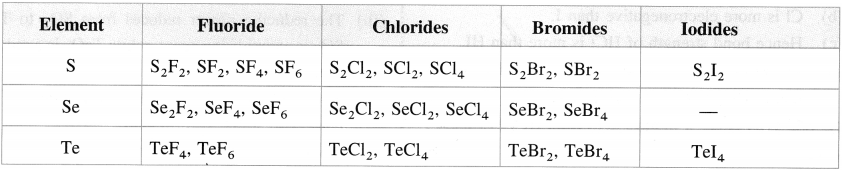
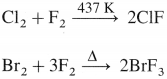


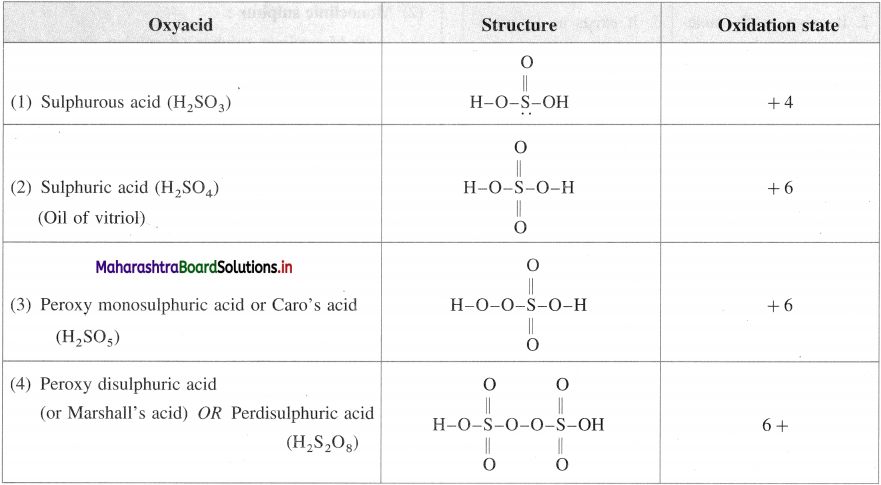

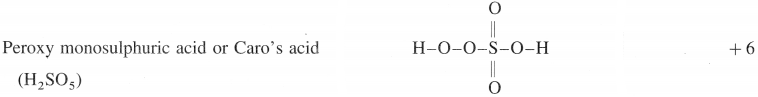
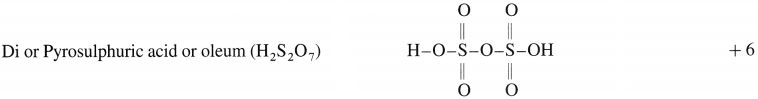
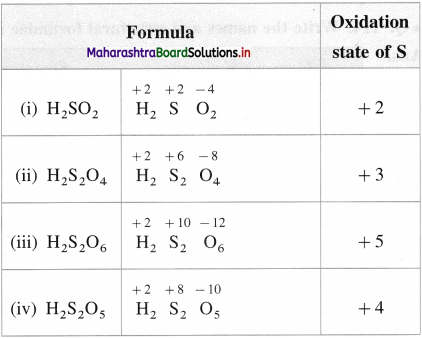


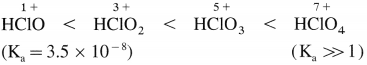
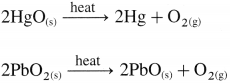






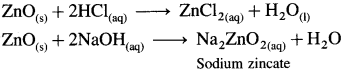
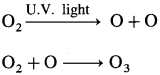




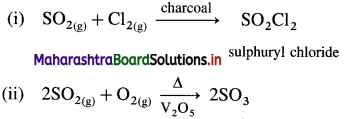


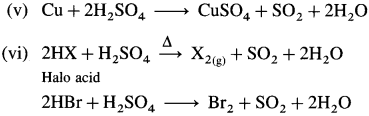

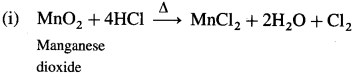





















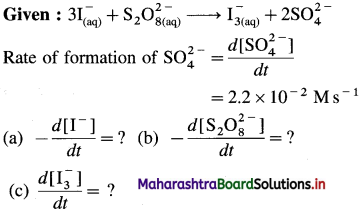






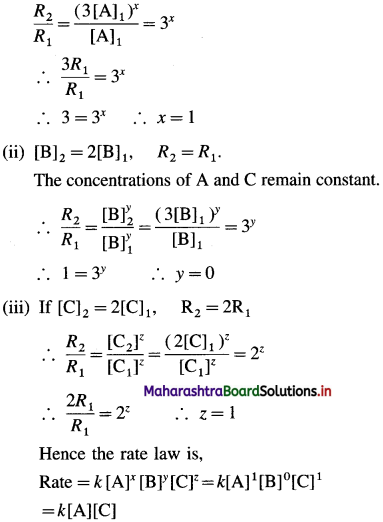
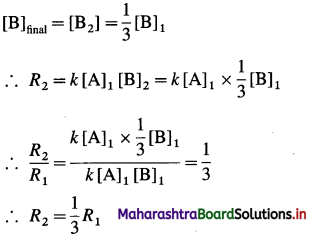

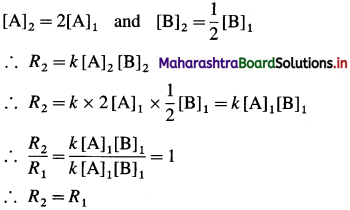
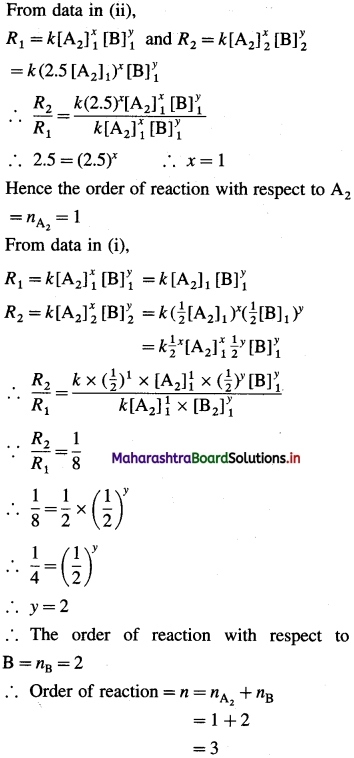

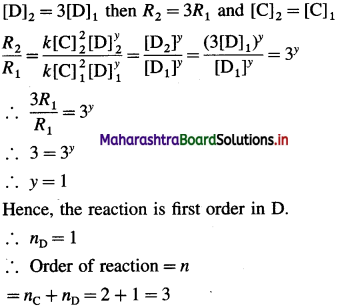


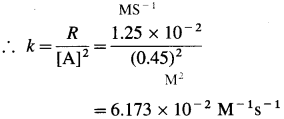




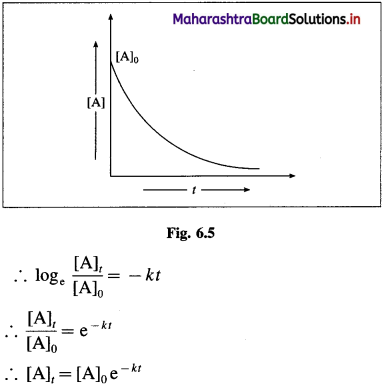
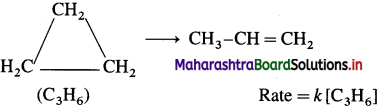
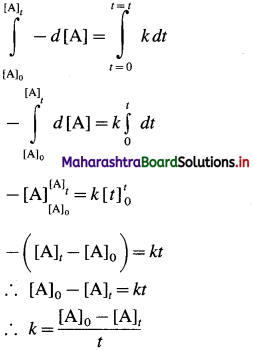




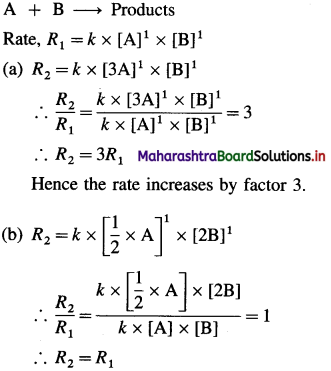

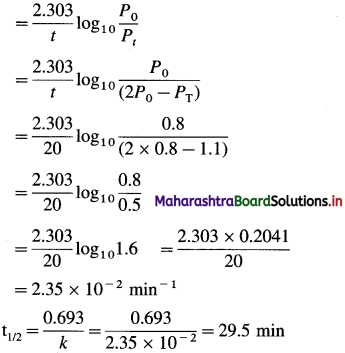
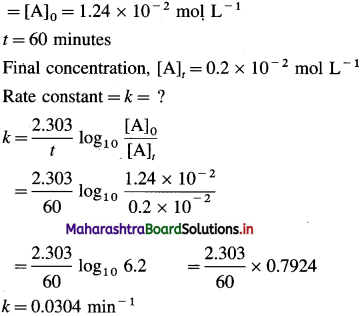




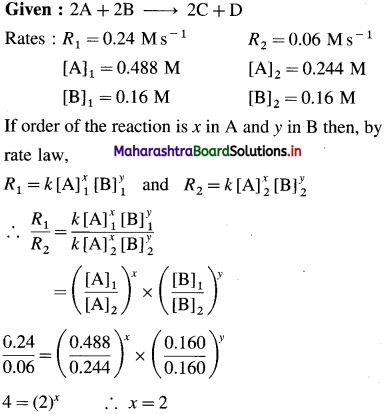
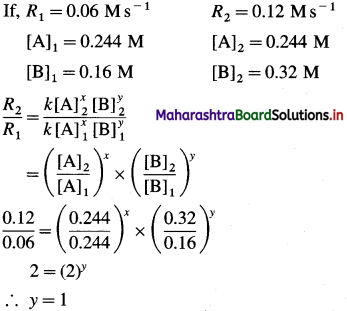
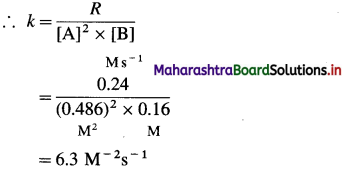





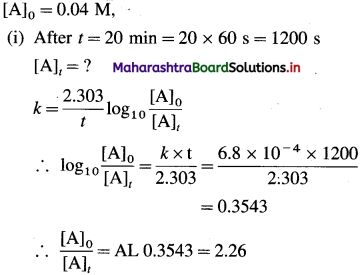

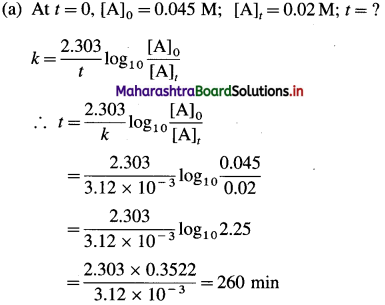
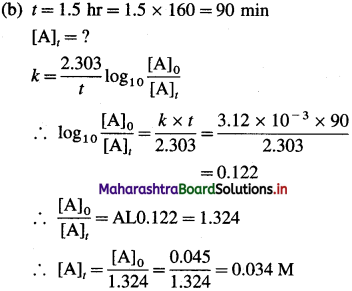

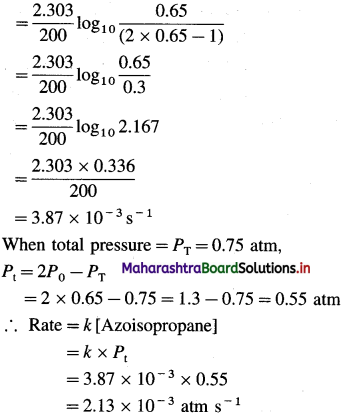




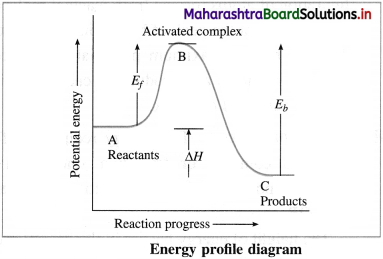
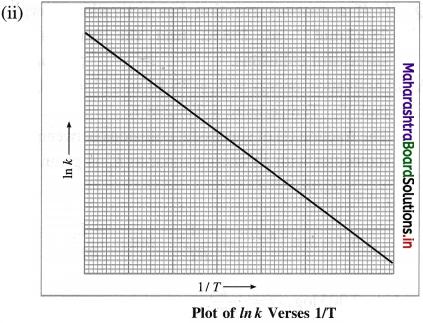



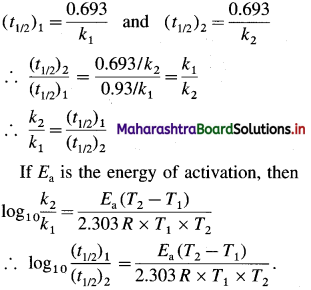
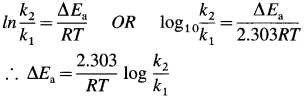

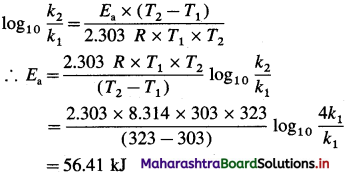

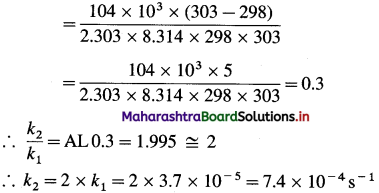
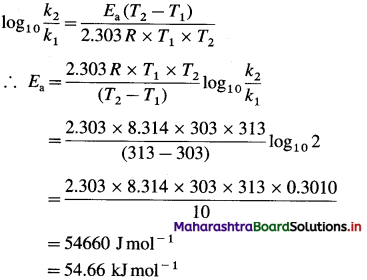

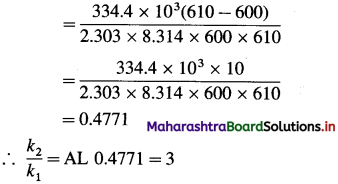

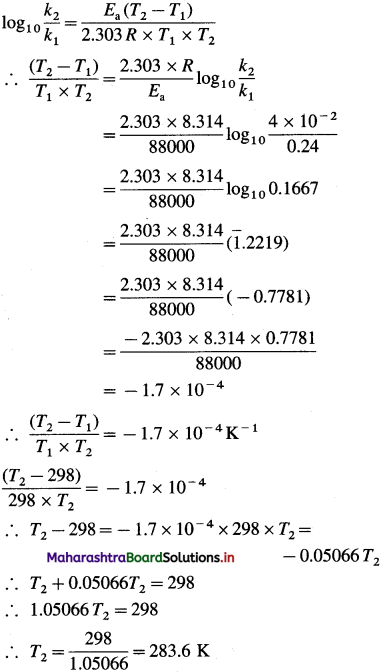

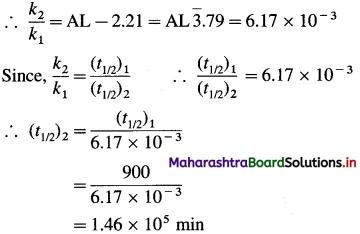



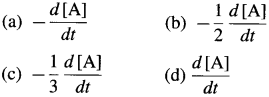
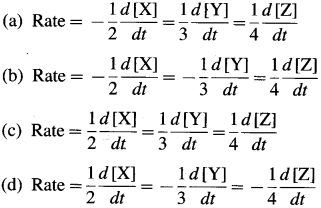
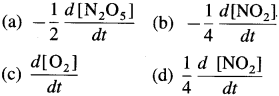


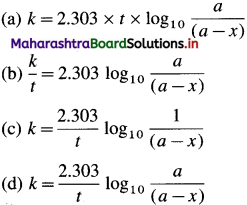

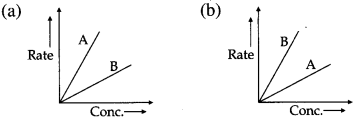
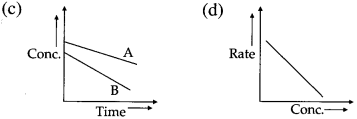


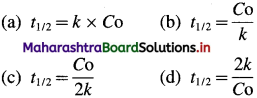



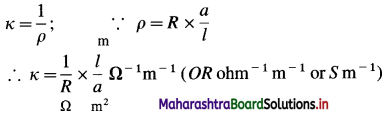
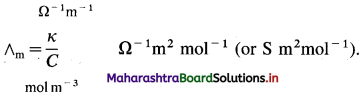
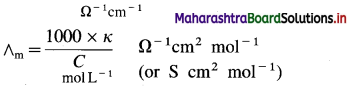
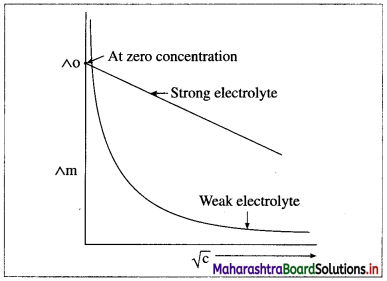
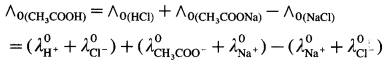
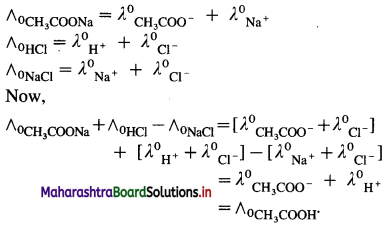
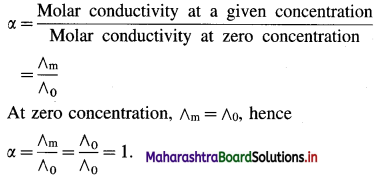
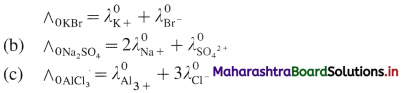
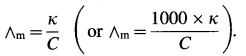
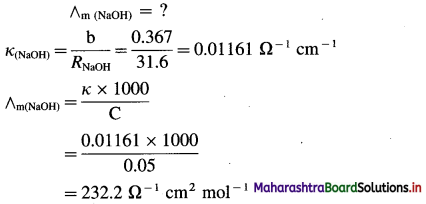
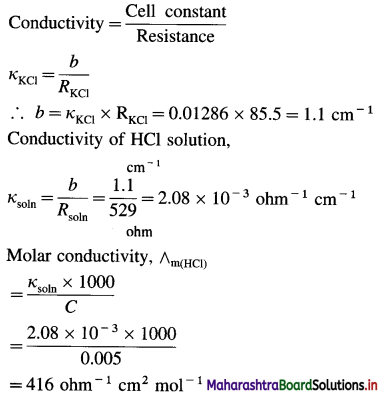
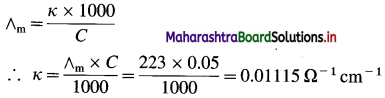
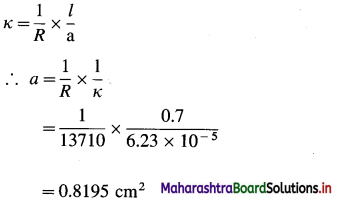
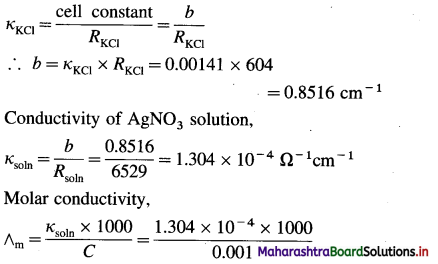
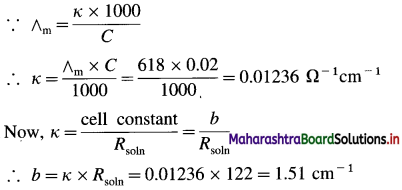
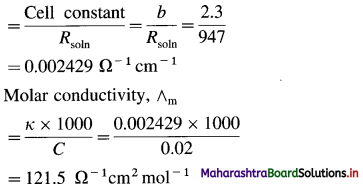
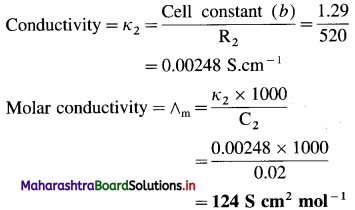
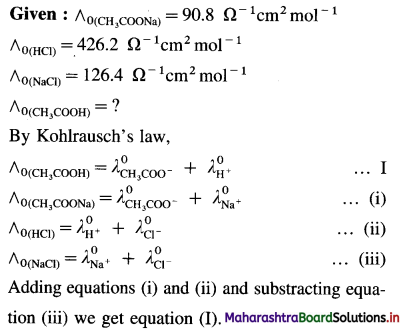

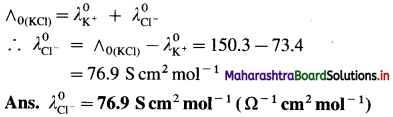
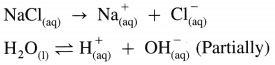
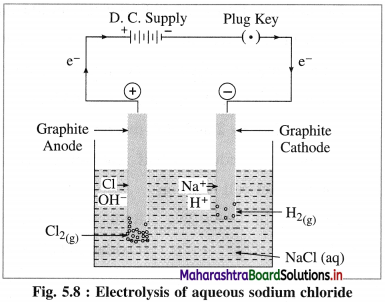

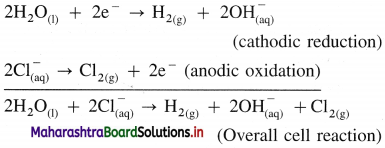
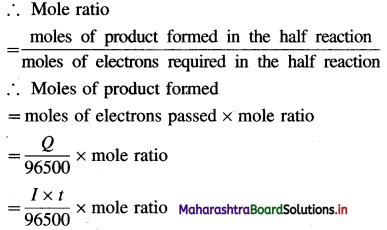

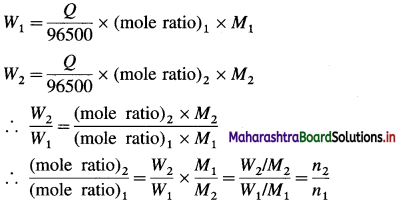
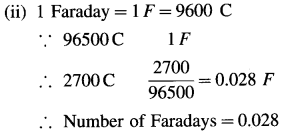
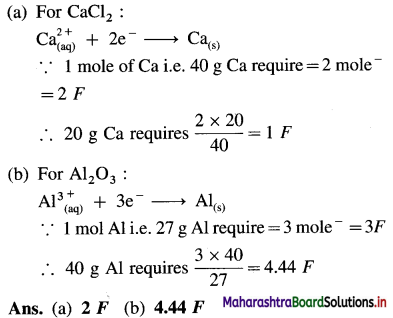
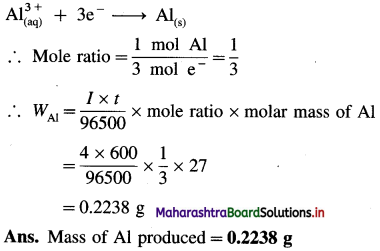
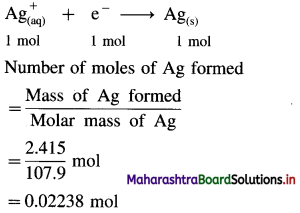
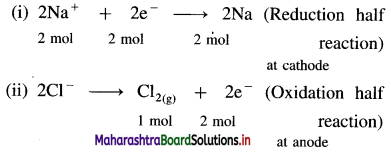
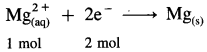
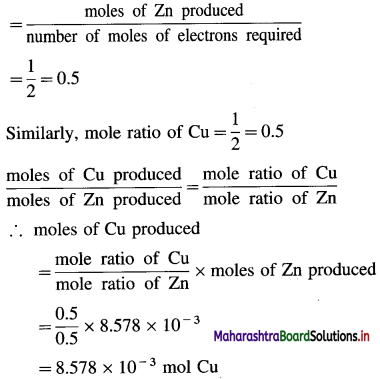
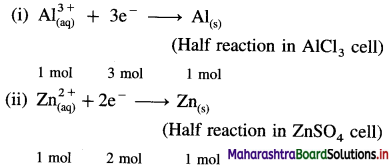
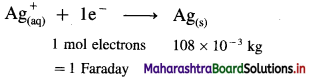
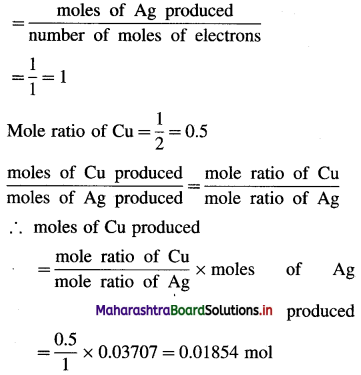
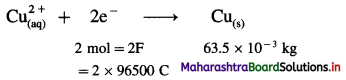
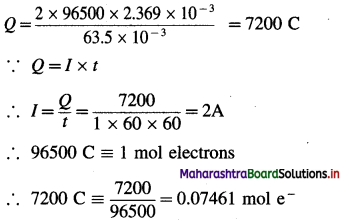
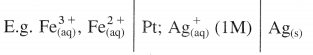
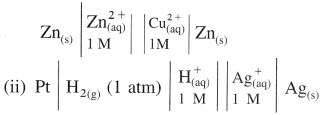
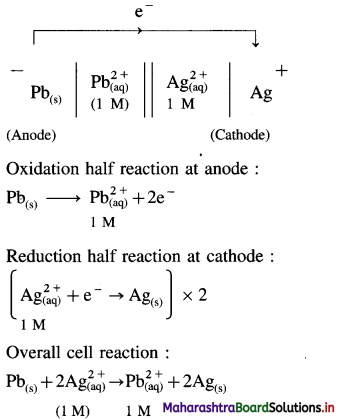
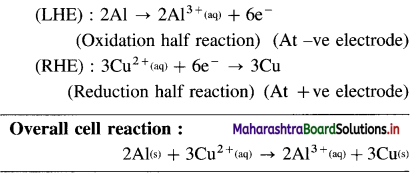
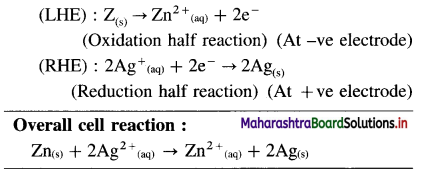
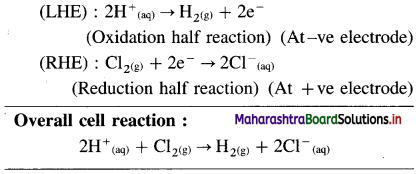

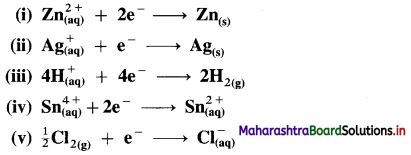
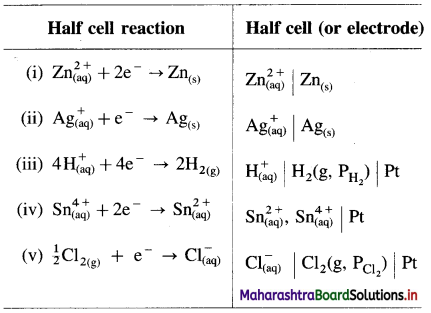
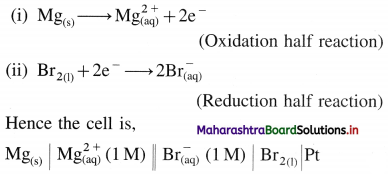
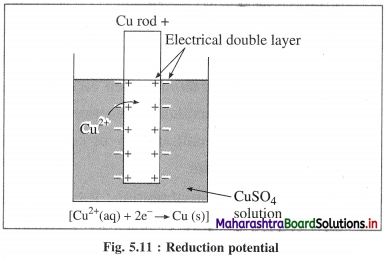

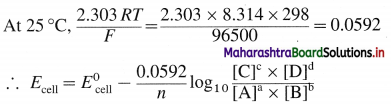
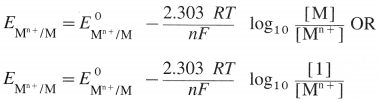
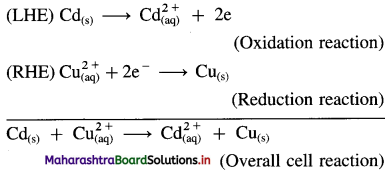

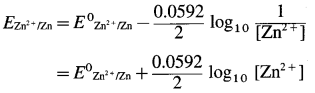
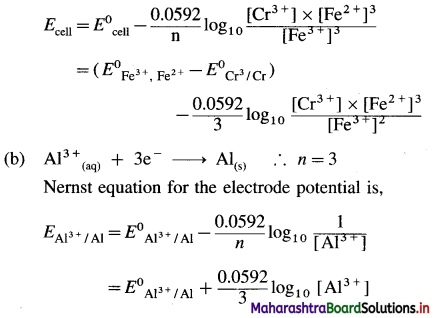
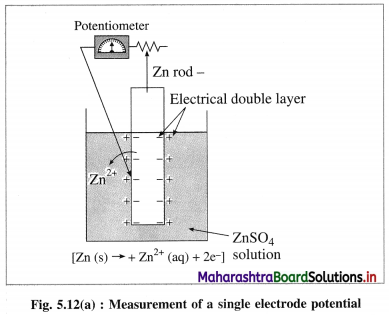
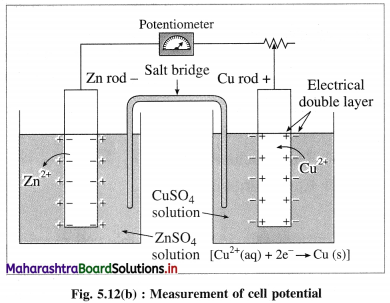
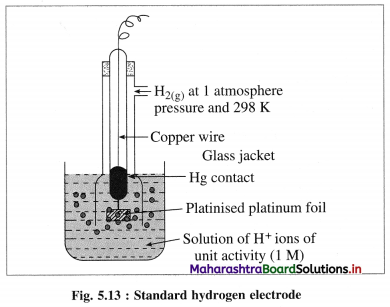
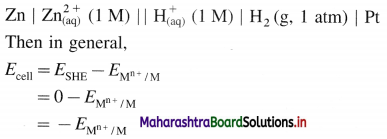

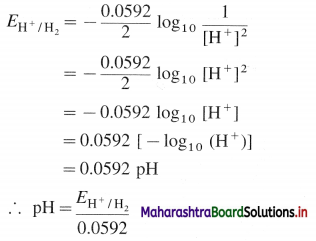
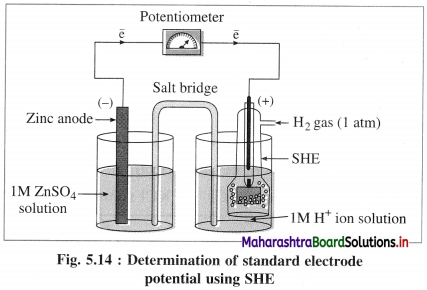
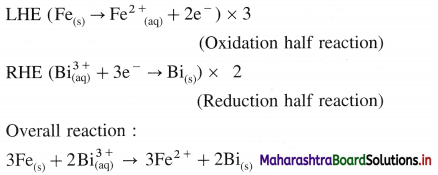
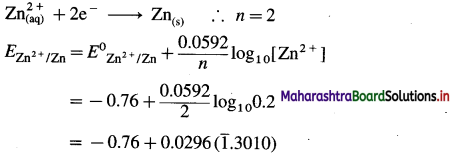
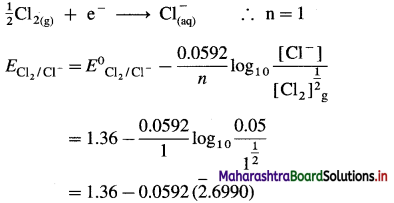
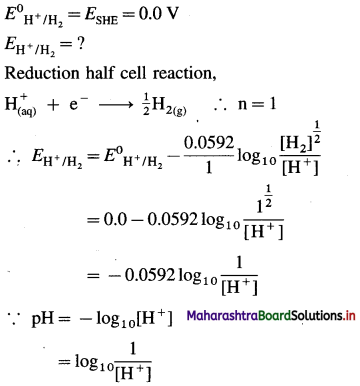
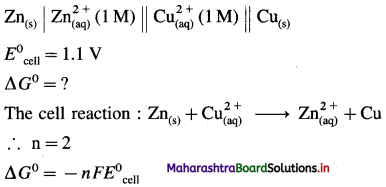
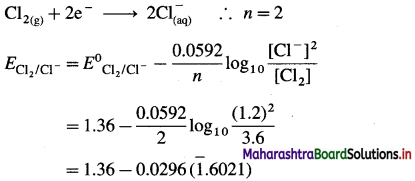
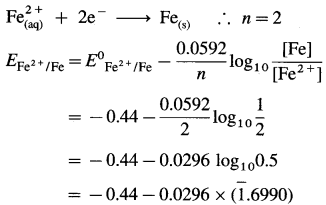
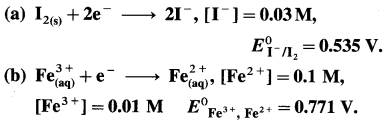
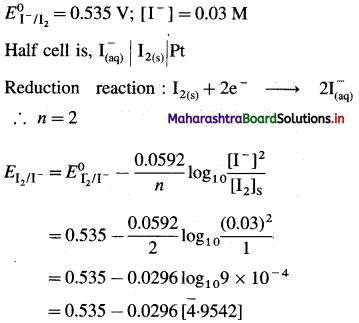
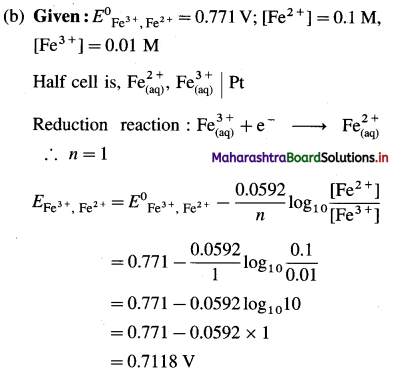
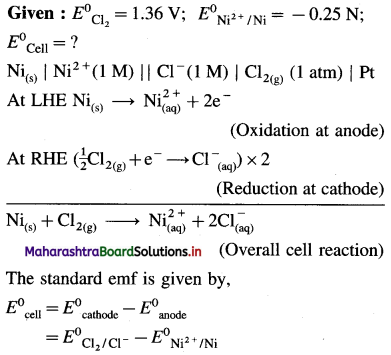
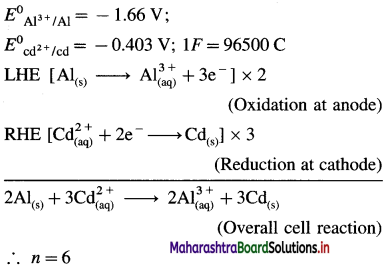
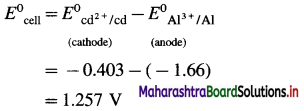
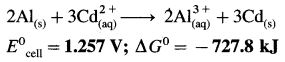
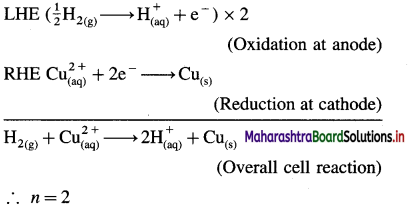

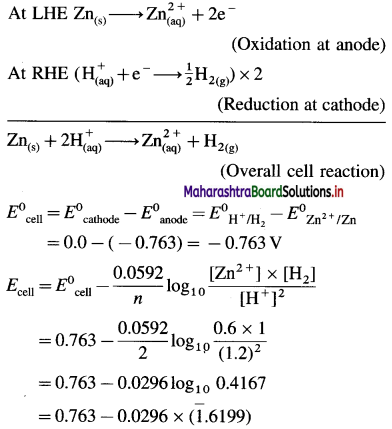

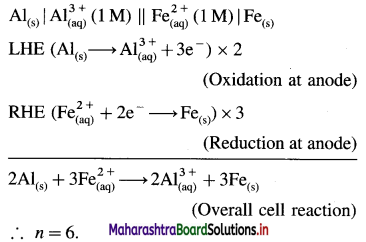

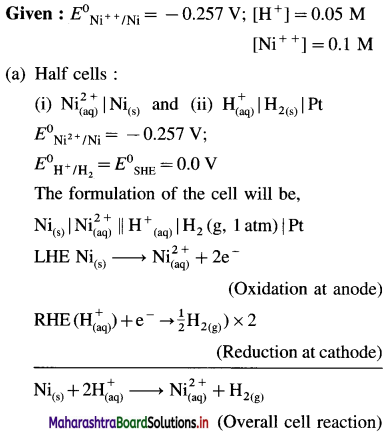
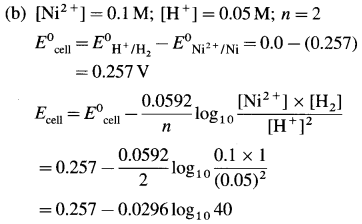

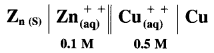
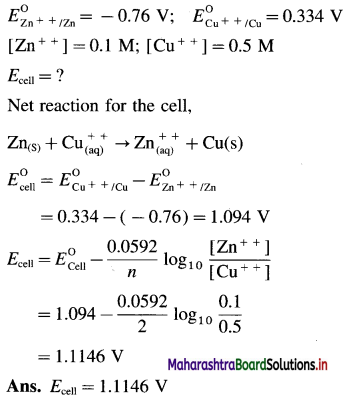
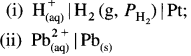
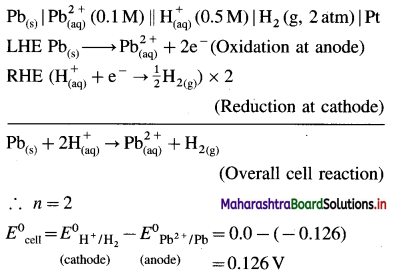
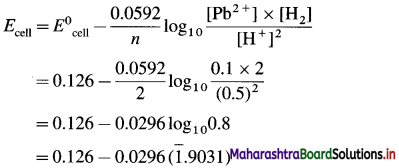
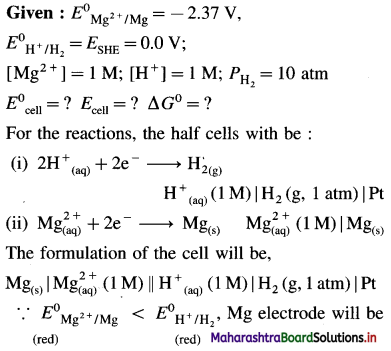
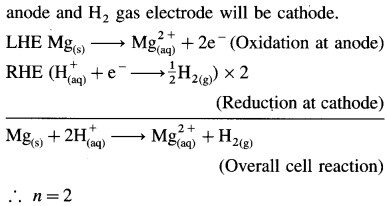
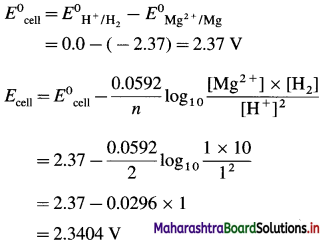
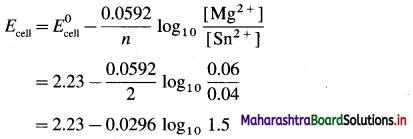
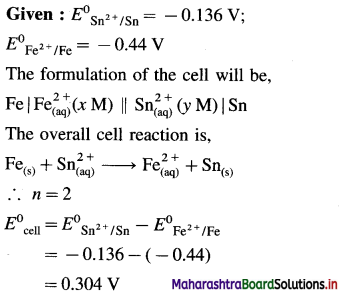
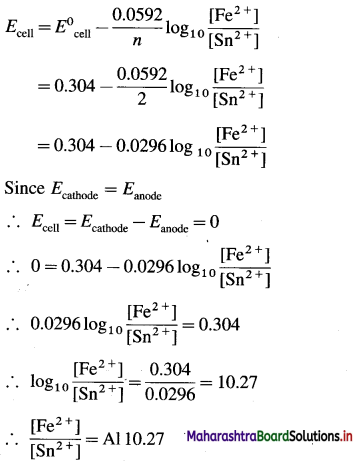
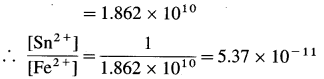
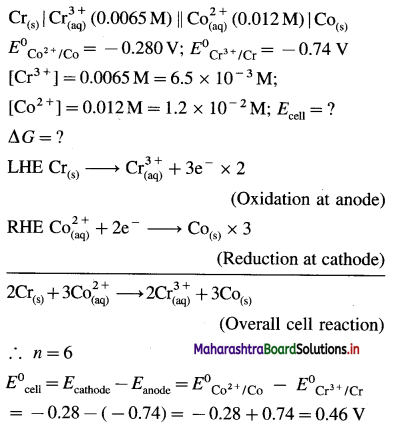
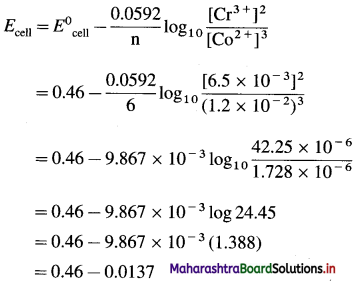
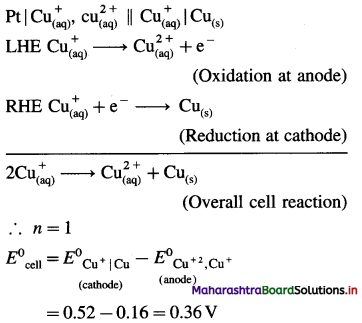
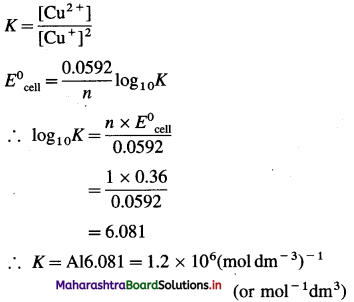
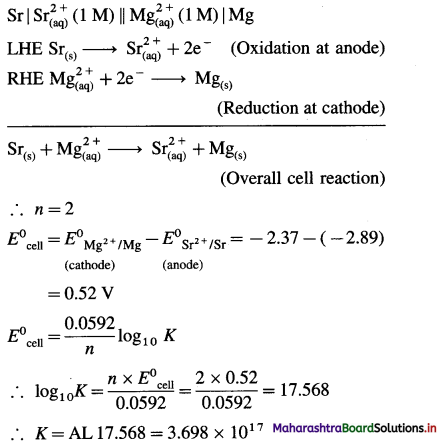
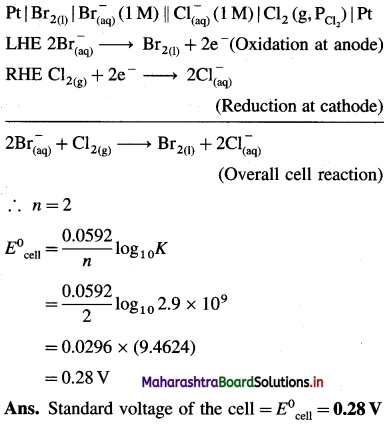

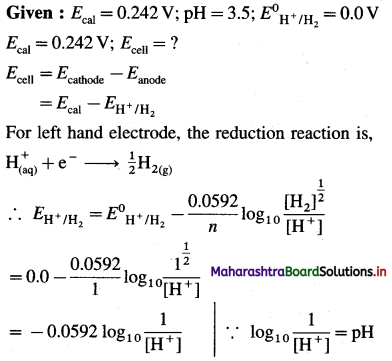
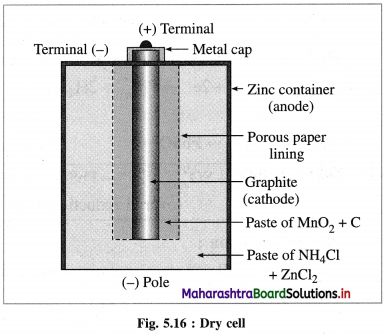
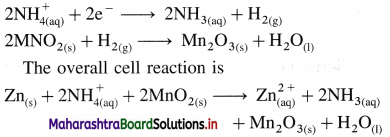
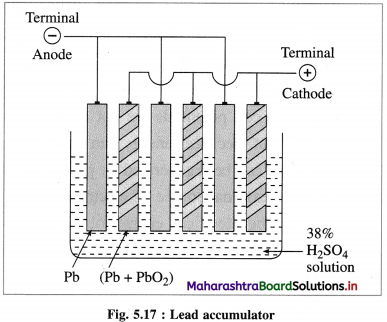
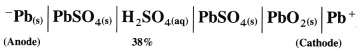
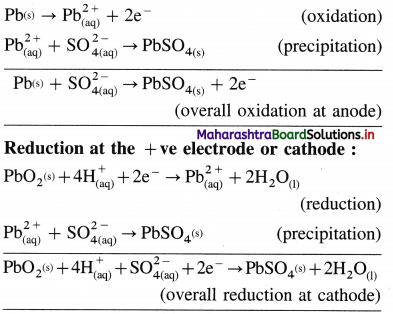
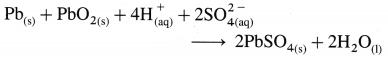
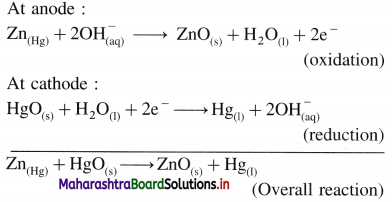
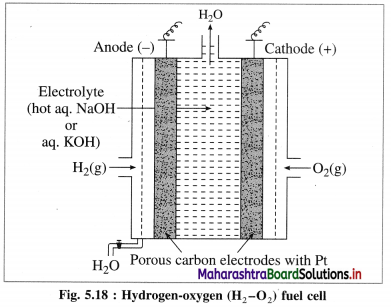
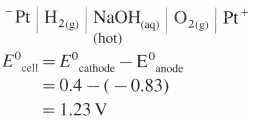
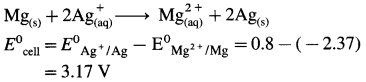
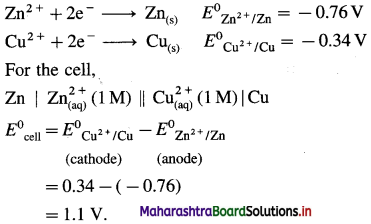
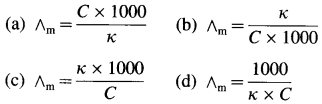
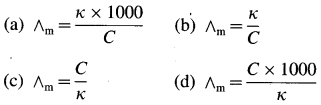
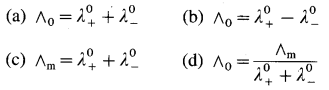

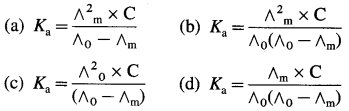

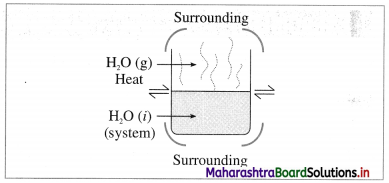
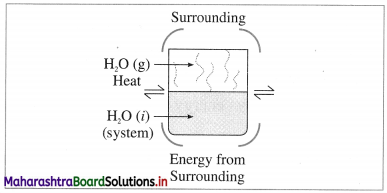
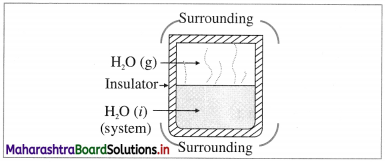
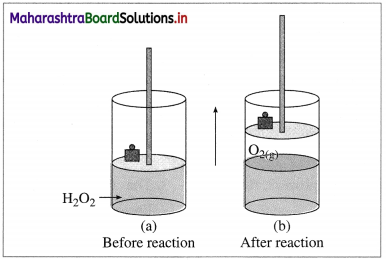
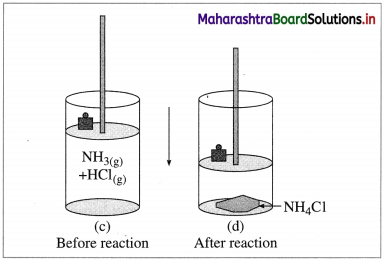
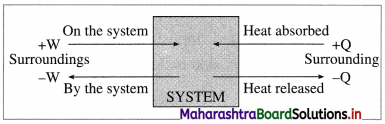
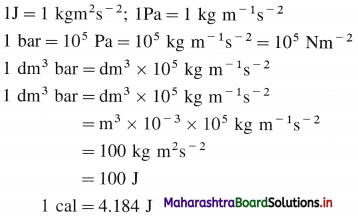
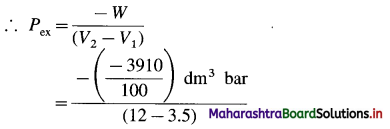
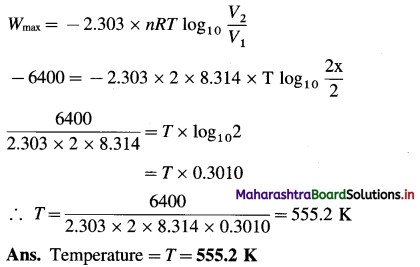
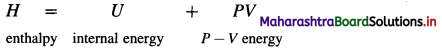
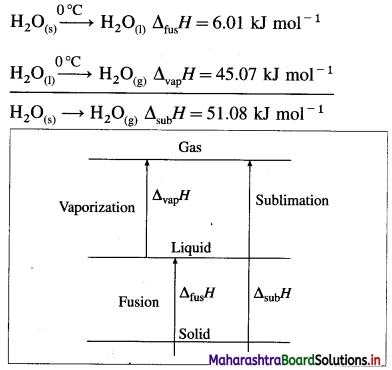
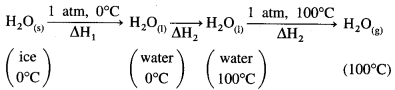
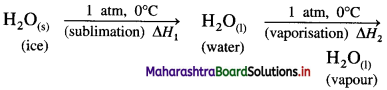
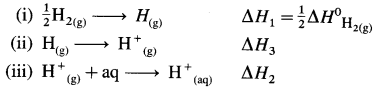

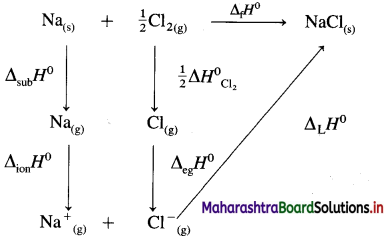
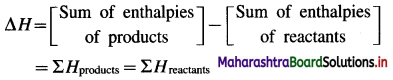
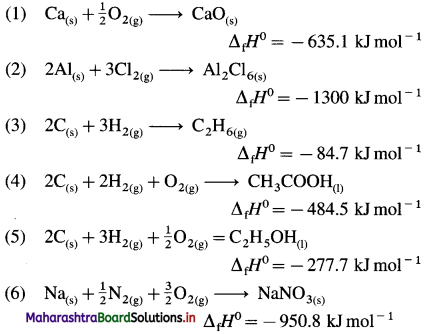
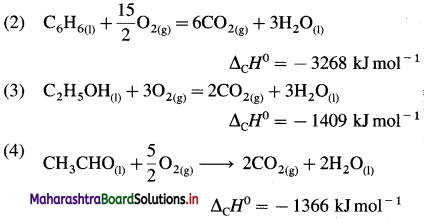
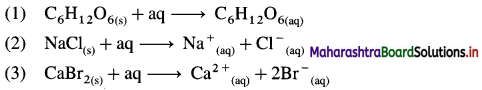
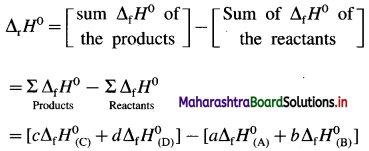
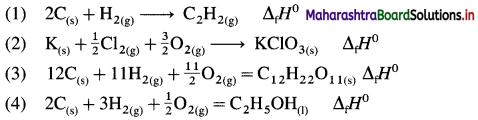
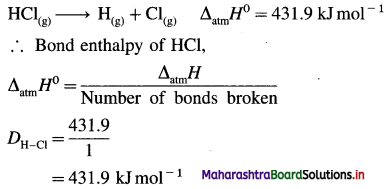
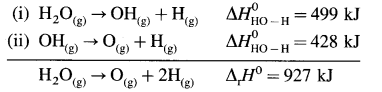
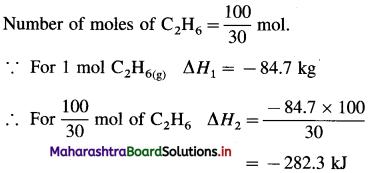
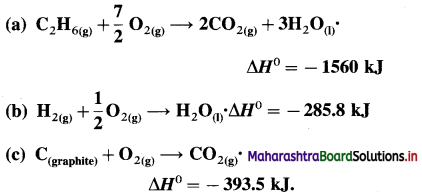
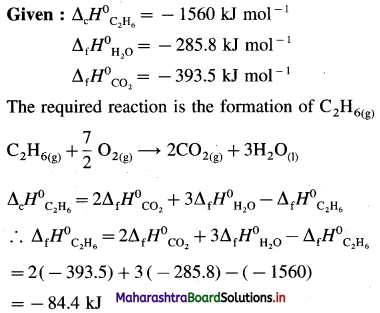

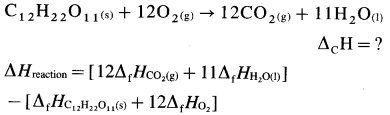
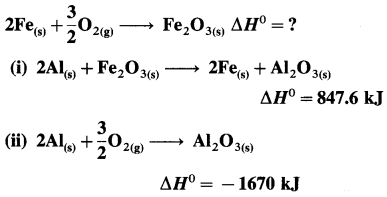
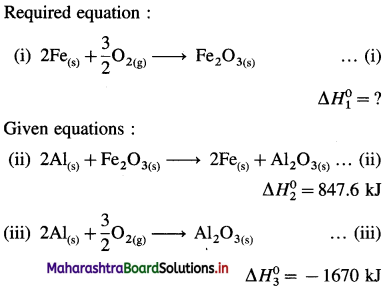
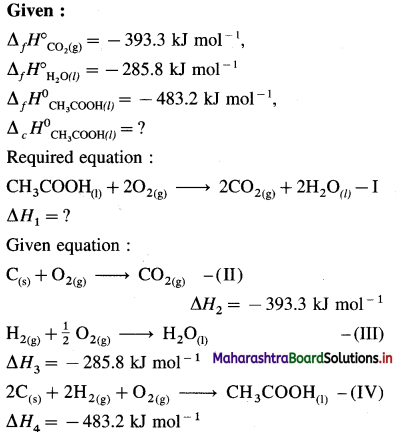

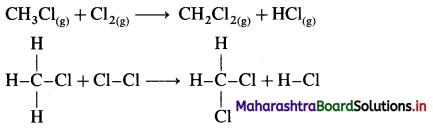
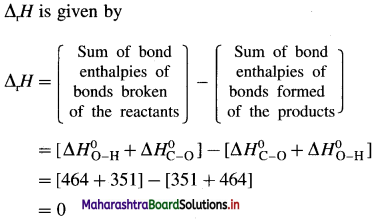
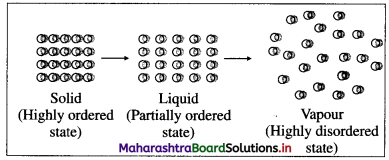
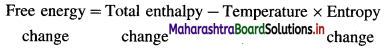
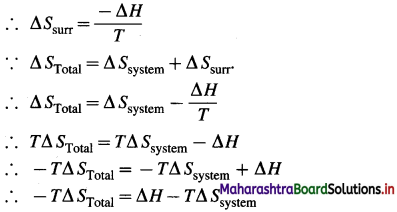
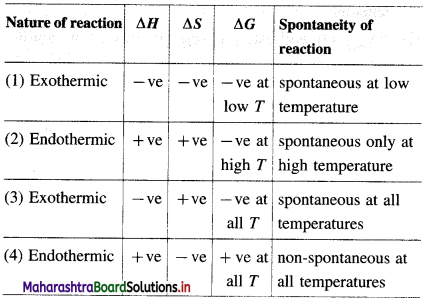
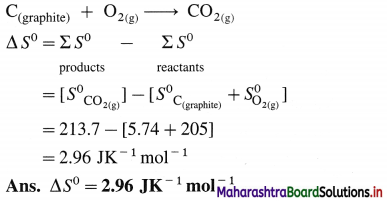
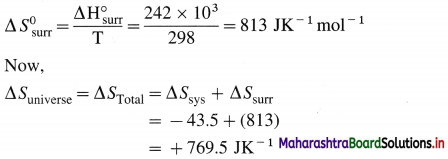

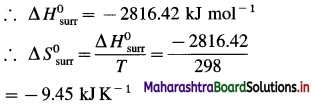
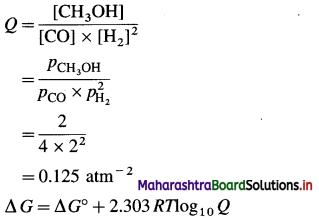
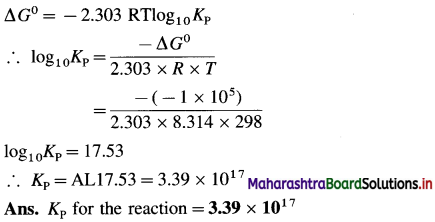
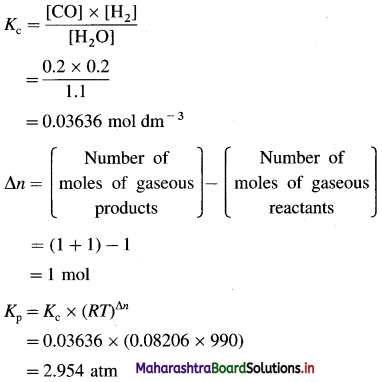
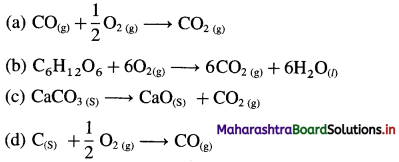
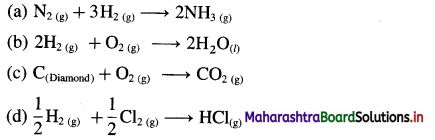
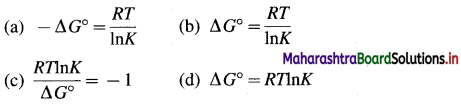

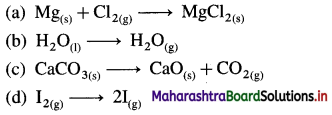
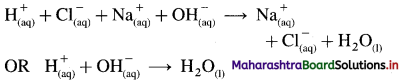
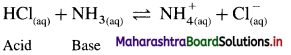
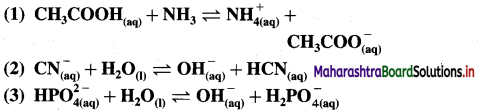
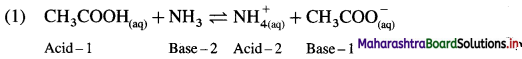
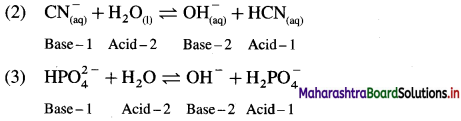

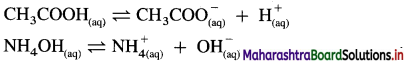
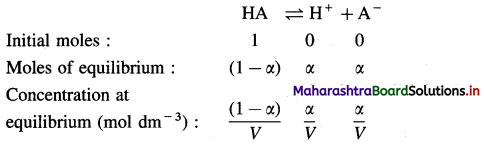
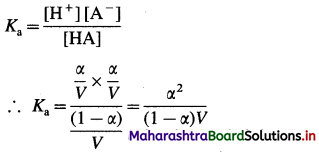
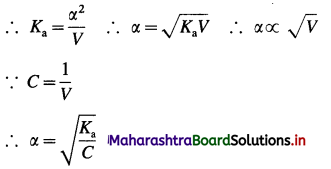
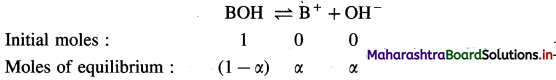
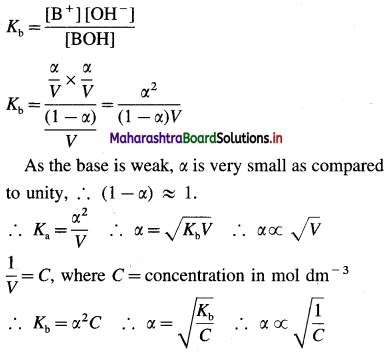
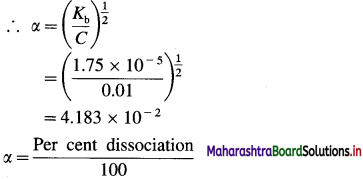
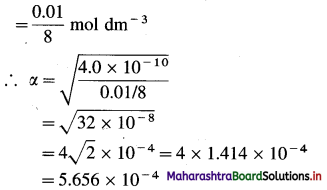
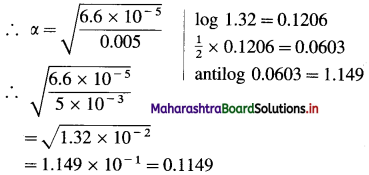
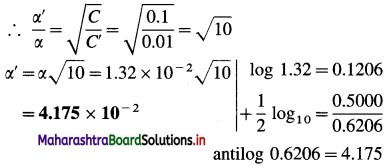
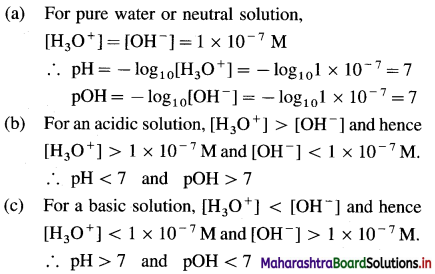
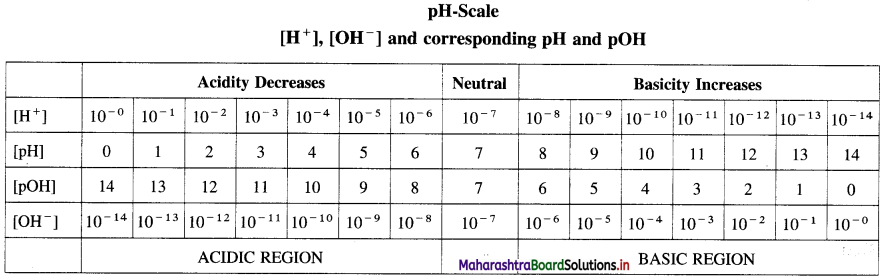
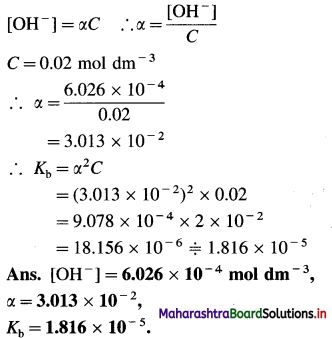
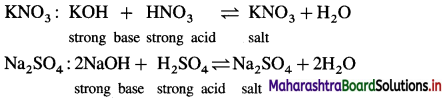
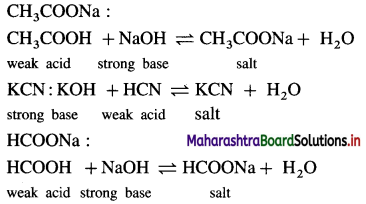
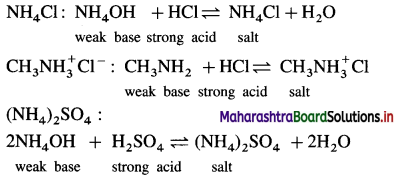
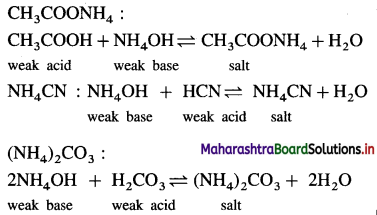
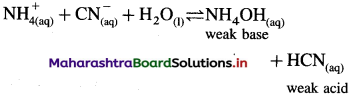
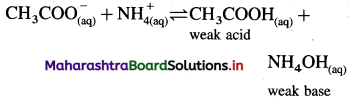
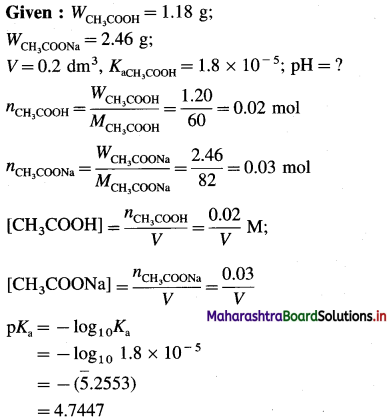
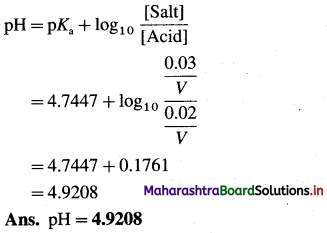
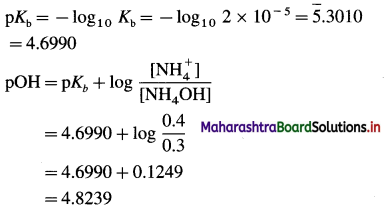


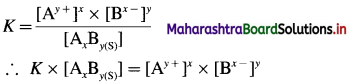
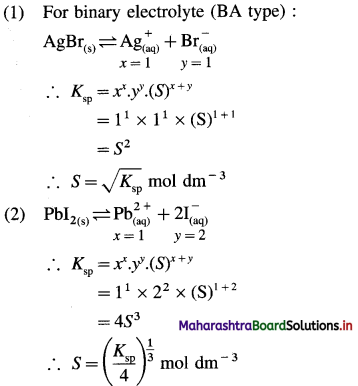
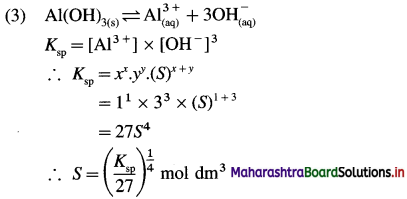
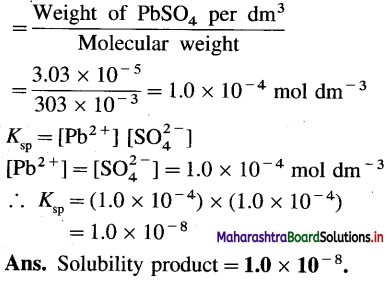
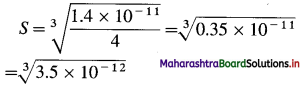
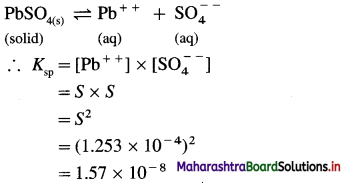
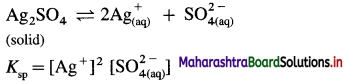
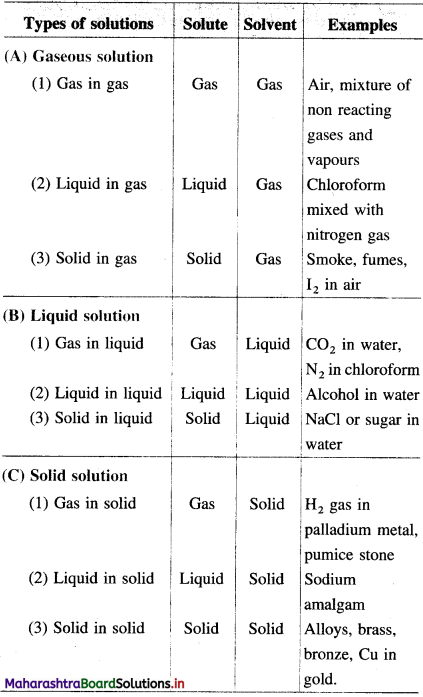

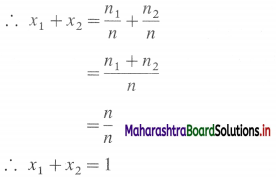

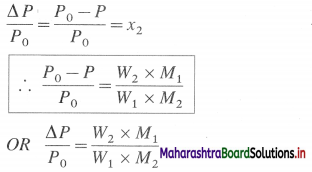

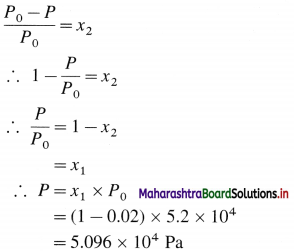
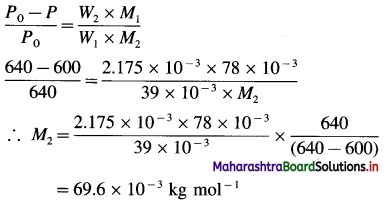
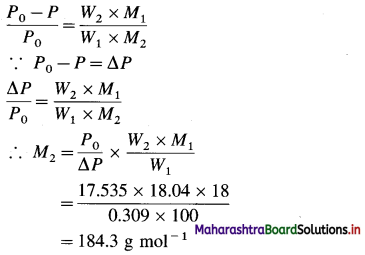
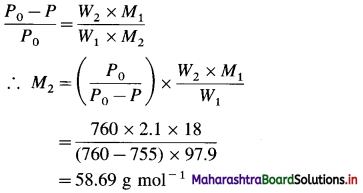
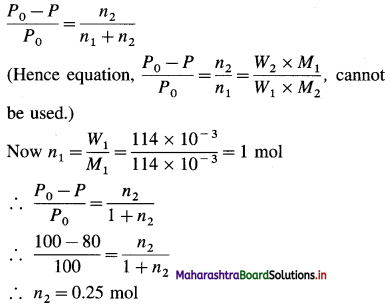
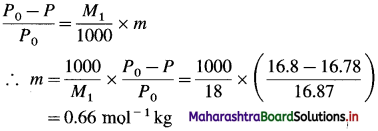

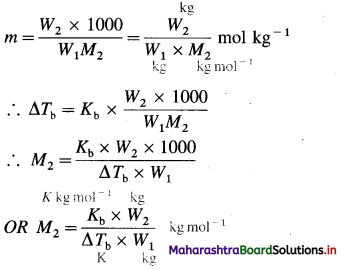
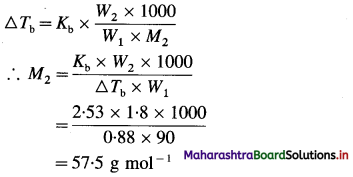
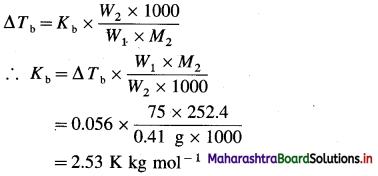
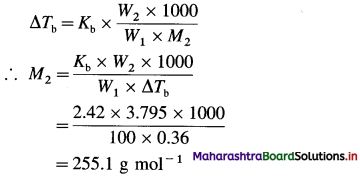
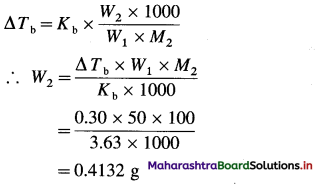
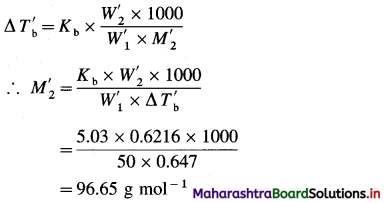
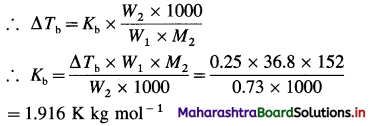
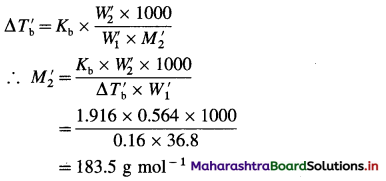
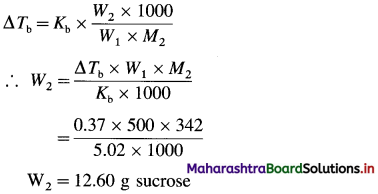
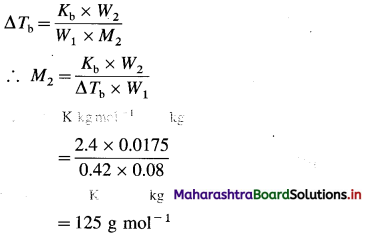
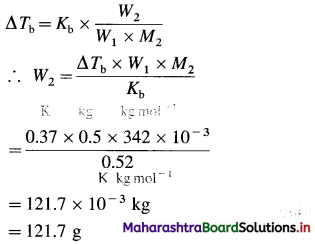

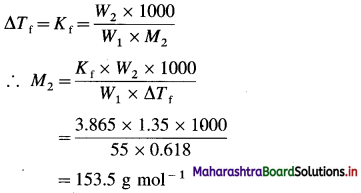
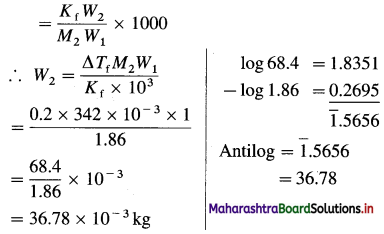
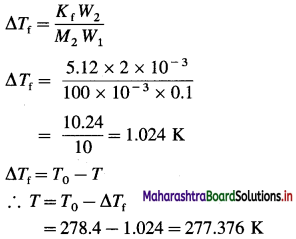
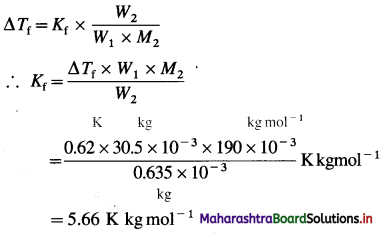
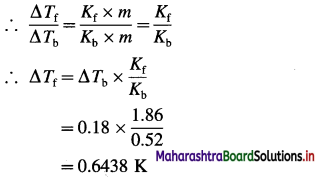
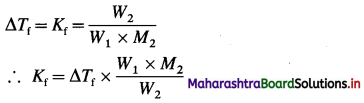
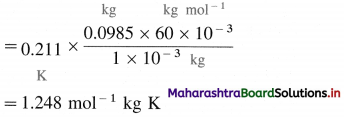
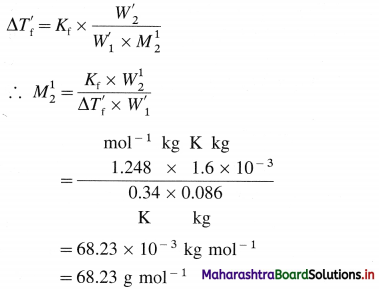
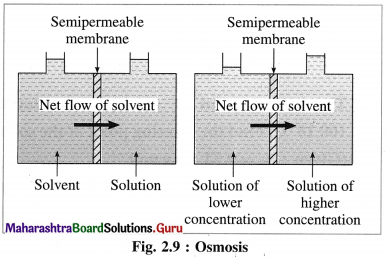

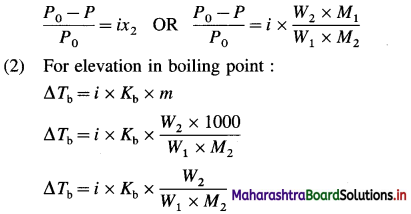
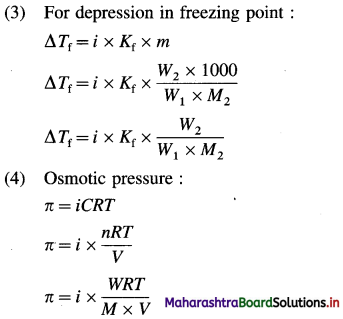
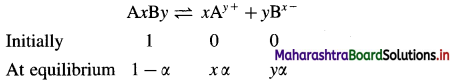
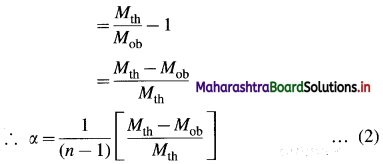
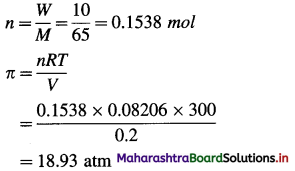
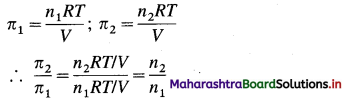
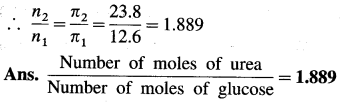

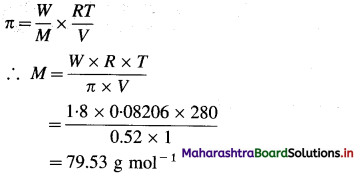
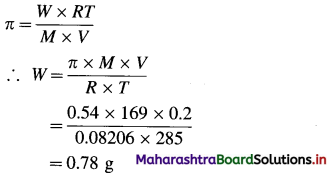
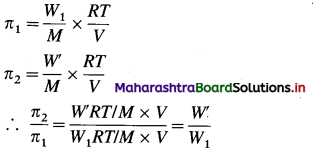
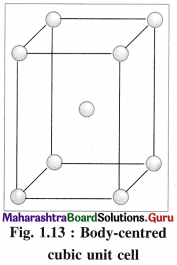
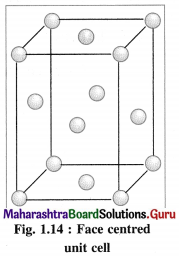
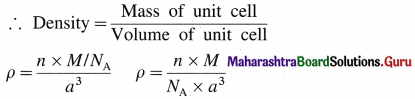
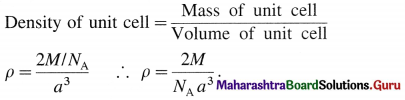
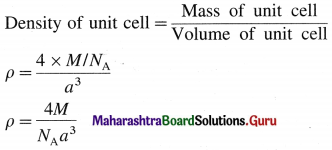
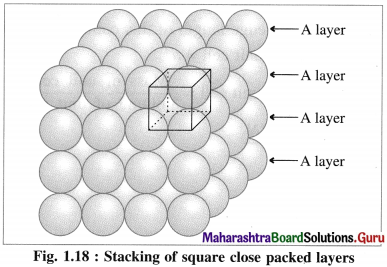
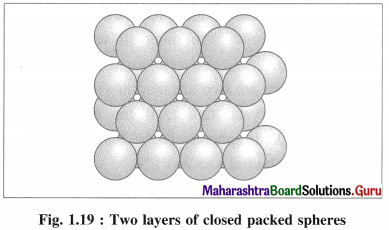
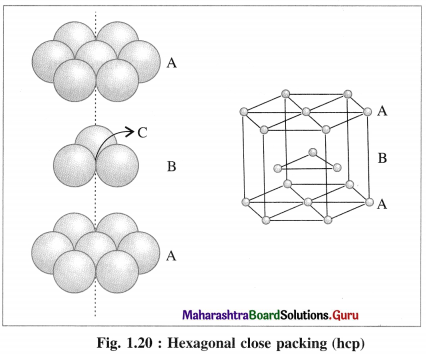
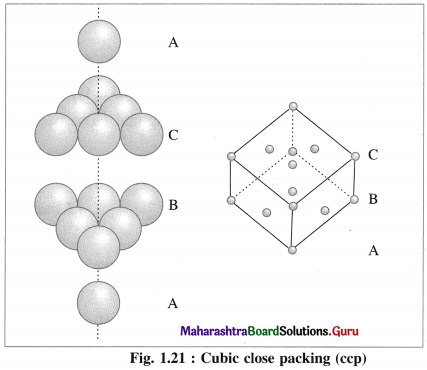


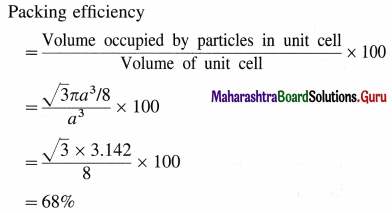
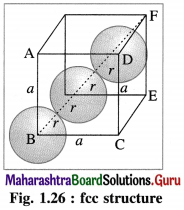
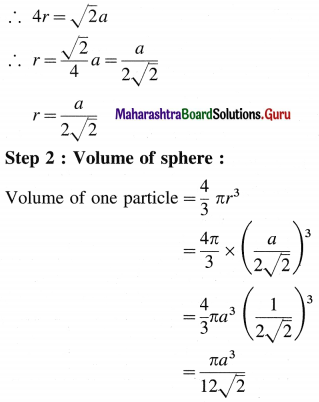
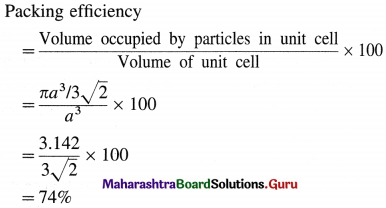
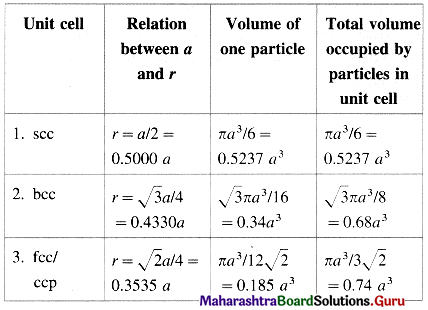

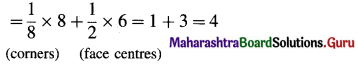
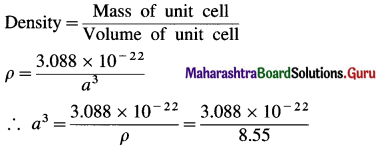
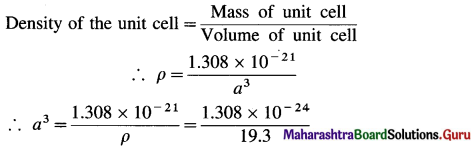

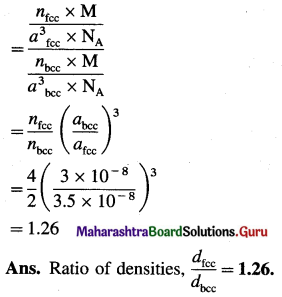
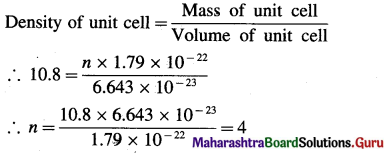
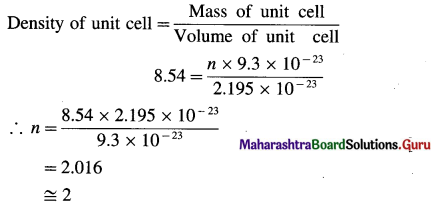
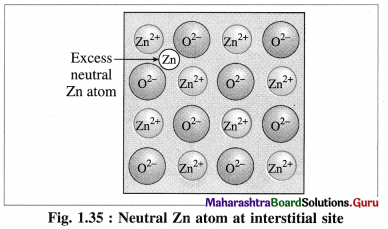
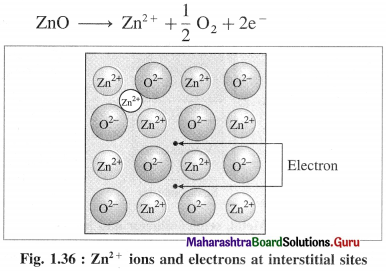
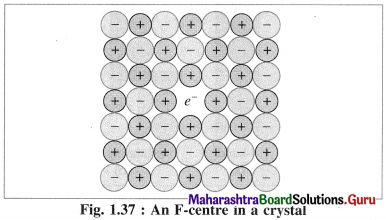
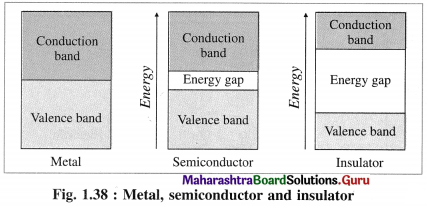

 linkage is present in terylene or dacron polymer.
linkage is present in terylene or dacron polymer.
 Repeating unit of natural rubber (Basic unit : isoprene)
Repeating unit of natural rubber (Basic unit : isoprene) and formaldehyde (HCHO)
and formaldehyde (HCHO)