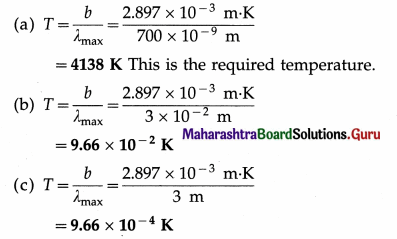
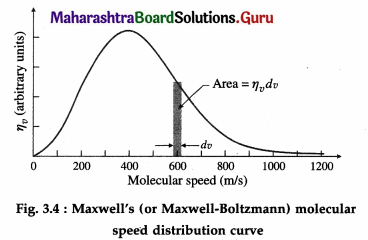
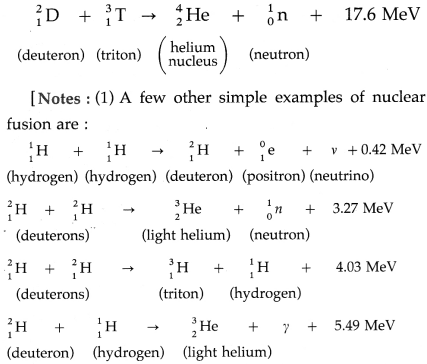
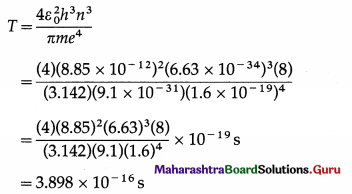
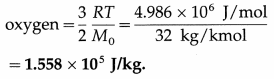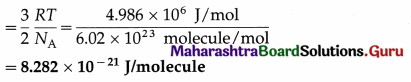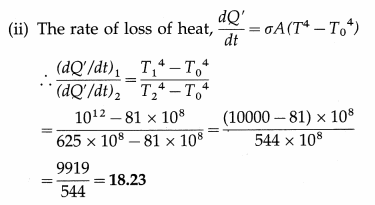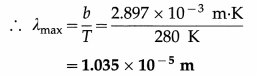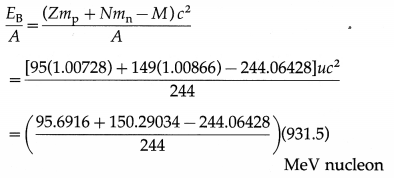Balbharti Maharashtra State Board Class 11 Sociology Important Questions Chapter 5 Culture Important Questions and Answers.
Maharashtra State Board 11th Sociology Important Questions Chapter 5 Culture
Choose the correct alternative and complete the statements.
Question 1.
The term ‘culture’ was used first by ……………….
(Bronislaw Malinowski / Edward Tylor / Max Weber)
Answer:
Edward Tylor
Question 2.
………………. culture is concrete and tangible in nature.
(Non-material / Folk / Material)
Answer:
Material
![]()
Question 3.
………………. culture refers to the ideas created by human beings.
(Material / Non-Material / Cognitive)
Answer:
Non-Material
Question 4.
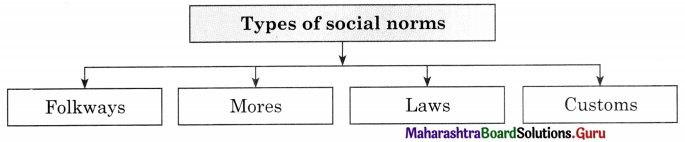
The ………………. aspects consist of folkways, mores, customs, conventions and laws.
(cognitive / normative / knowledge)
Answer:
normative
Question 5.
The ………………. aspects refer to understanding.
(normative / knowledge / cognitive)
Answer:
cognitive
Question 6.
The gap between material and non-material culture is known as ……………….
(cultural lag / language / folkways)
Answer:
cultural lag
Question 7.
………………. culture is considered as the epitome of the highest levels of human creativity.
(Folk / High / Mass)
Answer:
High
Question 8.
………………. culture refers to the culture of ordinary people.
(High / Folk / Popular)
Answer:
Folk
Question 9.
………………. culture is essentially a product of mass media.
(High / Popular / Mass)
Answer:
Mass
Question 10.
A ………………. is anything that is used to stand for something else.
(knowledge / symbol / language)
Answer:
Symbol
Question 11.
A group of words or ideas having common meaning is called ……………….
(language / value / symbol)
Answer:
Language
Question 12.
Direct knowledge is ………………. knowledge.
(mediated / immediate / indirect)
Answer:
Immediate
Question 13.
Indirect knowledge is ………………. knowledge.
(practical / logical / mediated)
Answer:
mediated
Question 14.
………………. involve standards of what is good or bad.
(Beliefs / Values / Language)
Answer:
Values
Question 15.
………………. are rules and behavioural expectation by which a society.
(Mores / Norms / Values)
Answer:
Norms
Question 16.
………………. are mildly enforced social expectations.
(Mores / Language / Folkways)
Answer:
Folkways
Question 17.
………………. are strictly held beliefs about behaviours.
(Mores / Values / Knowledge)
Answer:
Mores
Question 18.
Migration and ………………. leads to a mixing of culture.
(globalization / traditions / customs)
Answer:
globalisation
Question 19.
………………. is a sum-total of the ideal patterns and norms of behaviour of a group.
(Values, Folkways, Culture)
Answer:
Culture
Question 20.
………………. is one of the most important vehicles for perpetuating cultural patterns.
(Customs / Language / Symbols)
Answer:
Language
![]()
Question 21.
………………. activities bring people together and build social solidarity.
(Social / Cultural / Political)
Answer:
Cultural
Question 22.
………………. is the view that one’s own culture is better than anyone else’s culture.
(Relativism / Ethnocentrism / Hybridisation)
Answer:
Ethnocentrism
Question 23.
Cultural ………………. refers to the parts of one culture get recombined with the cultures of another.
(hybridisation / relativism / ethnocentrism)
Answer:
hybridisation
Question 24.
Interaction of global process with local processes is known as ……………….
(glocalisation / globalization / migration)
Answer:
glocalisation
Question 25.
………………. and globalisation leads to a mixing of cultures.
(Industrialisation / Migration / Digitisation)
Answer:
Migration
Correct the incorrect pair.
Question 1.
(a) The Indian flag — Symbol
(b) Norms and values – Non-material culture
(c) Recorded pop music – Popular culture
(d) Computers and airplanes – Material culture
Answer:
(c) Recorded pop music – Mass culture
Question 2.
(a) Direct knowledge Immediate knowledge
(b) Folkways – Mildly enforced
(c) Mores – Serious norms
(d) Norms – Group of ideas
Answer:
(d) Norms – Behavioural expectation
Question 3.
(a) Globalisation – Global restaurant chain
(b) Arts – Identity formation
(c) Emojis / Smileys – Symbols
(d) Normative – Ideas and beliefs
Answer:
(d) Normative – Folkways and mores.
Question 4.
(a) Aesthetically superior culture – High culture
(b) Culture of ordinary people – Folk culture
(b) Product of mass media – Mass culture
(b) No cultural expertise Sub-culture
Answer:
(d) No cultural expertise – Popular culture
Question 5.
(a) What we shouldn’t do – Proscriptive norms
(b) What we should do – Prescriptive norms
(c) Strictly held beliefs – Mores
(d) Mildly enforce – Customs
Answer:
(d) Mildly enforced – Folkways
Identify the appropriate term from the given options.
(Symbols, High Culture, Folk Culture, Mass Culture, Prescriptive Norms Popular Culture, Subculture, Values, Folkways, Language)
Question 1.
Culture shared by ethnic group.
Answer:
Subculture
![]()
Question 2.
Bhangada in Punjab.
Answer:
Folk Culture
Question 3.
Poems of Kabir Das.
Answer:
High Culture
Question 4.
Emojis / Smileys.
Answer:
Symbol
Question 5.
Desirable or Undesirable
Answer:
Values
Question 6.
Customary and habitual ways of life.
Answer:
Folkways
Question 7.
It is the method to mould behaviour.
Answer:
Language
Question 8.
Parents expect obedience from children.
Answer:
Prescriptive Norms
Correct underlined words and complete the sentence.
Question 1.
Possession of culture distinguishes animals from non-humans.
Answer:
Possession of culture distinguishes humans from non-humans.
Question 2.
Culture is the social legacy the individual acquired from one’s ability.
Answer:
Culture is the social legacy the individual acquired from one’s group.
Question 3.
Non-material culture are man-made objects.
Answer:
Material culture are man-made objects.
![]()
Question 4.
The Non-material aspect of culture changes very fast.
Answer:
The material aspect of culture changes very fast.
Question 5.
Some symbols are types of verbal communication.
Answer:
Some symbols are types of non-verbal communication.
Question 6.
Knowledge is the chief vehicle of culture.
Answer:
Language is the chief vehicle of culture.
Question 7.
Values are statements that people hold to be true.
Answer:
Beliefs are statements that people hold to be true.
Question 8.
Folkways are more serious norms.
Answer:
Mores are more serious norms.
Question 9.
Mores are customary practices.
Answer:
Folkways are customary practices.
Question 10.
Culture is learnt through globalization.
Answer:
Culture is learnt through socialization.
Question 11.
The use of mobile phones has brought significant changes in traditional customs of communication.
Answer:
The use of mobile phones has brought significant changes in traditional etiquettes of communication.
Question 12.
Participation in the debates can promote inter cultural understanding.
Answer:
Participation in the arts can promote inter cultural understanding.
Question 13.
Cultural activities foster social exclusion.
Answer:
Cultural activities foster social inclusion.
Question 14.
Relativism consists of evaluating other culture from the perspective of one’s own.
Answer:
Ethnocentrism consists of evaluating other culture from the perspective of one’s own.
Question 15.
Arrogance is positive side of ethnocentrism.
Answer:
Arrogance is negative side of ethnocentrism.
Question 16.
Confidence and assurance to the culture is a negative side of ethnocentrism.
Answer:
Confidence and assurance to the culture is a positive side of ethnocentrism.
![]()
Question 17.
Language mixing, fusion music are examples of globalisation.
Answer:
Language mixing, fusion music are examples of cultural hybridisation.
Question 18.
The first element that exists in every culture is a variety of language.
Answer:
The first element that exists in every culture is a variety of symbols.
Write suitable examples of given concepts and justify your answer.
Question 1.
Cultural hybridisation.
Answer:
Example : Today we prefer Italian pizza with tandoori paneer as a topping which indeed is very Indian. It is convergence of Italian and Indian culture of food by modifying menus. Celebration of Valentine’s Day, language mixing, fusion music are examples of cultural hybridisation.
- Cultural hybridization refers to the ways in which parts of one culture get recombined with the cultures of another.
- Cultural hybridization is a universal process which is seeded up through globalization.
- In the globalization process, some forms of new and different cultural practice or behaviour develops from the mixing of different cultural traditions. The groups do not necessarily give up their own but participate in various ways in each other’s cultural activities. We see hybridization in many aspects of culture.
Question 2.
Ethnocentrism.
Answer:
Example : Throughout Asia the way of eating is to use chopsticks with every meal. These people may find that people in other societies using forks, spoons, knives, etc. to eat are foolish. Demeaning other cultures can enhance one’s feeling of pride in their own culture.
- Ethnocentrism is the view that one’s own culture is better than anyone else’s culture.
- It consists of evaluating other cultures from the perspective of one’s own.
- It refers to the tendency to assume that one’s own culture and way of life are superior to all others.
- The ethnocentric person sees his or her own culture as the most important.
Question 3.
Glocalisation.
Answer:
Example : A global restaurant chain modifying their menus based on the unique culture they are in but maintaining their brand. Indian spices are incorporated in the differently flavoured burger of McDonald’s.
- Glocalisation is a combination of the words “globalisation” and “localisation”, used to describe a product or service that is developed and distributed globally,
- Glocalisation is a global process interacting with local processes.
- In the contemporary society, global styles are given a unique flavour.
Question 4.
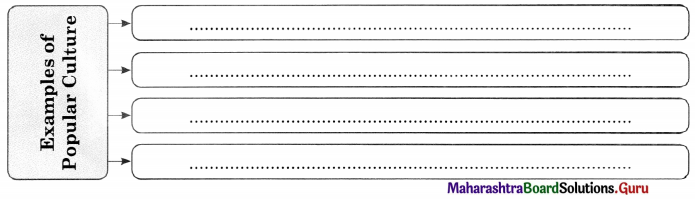
Popular Culture.
Answer:
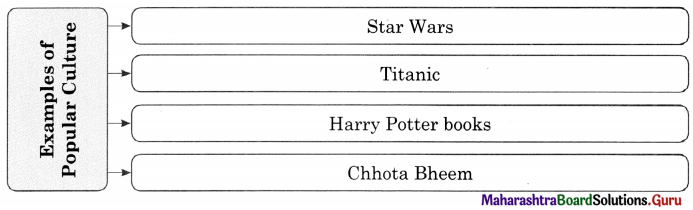
Example : Some sporting events, such as World Cup and Olympics are enjoyed by a world community. For example, mass market films such as Star Wars or Titanic, Harry Potter books, Chandoba, Chhota Bheem etc.,
Popular culture includes any cultural product appreciated by a large number of ordinary people with no great pretentions of cultural expertise.
![]()
Write short notes.
Question 1.
Types of Culture.
Answer:
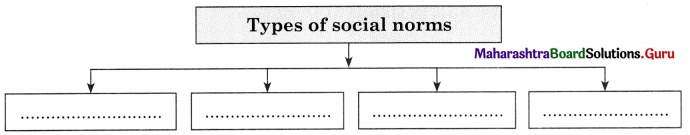
Culture is divided into two types :
1. Material Culture : Material culture are man-made. These are concrete and tangible in nature. It consists of manufactured objects like clothing, roads, jewellery, computers, airplanes, television, etc.
2. Non-material Culture : Non-material culture refers to the ideas created by human beings. The nature of non-material culture is abstract and intangible. For example, norms, values, signs and symbols, knowledge, beliefs etc. Non-material culture is further divided into cognitive and normative aspects of culture. The material aspect of culture changes very fast.
It is convenient or easy to adapt to new fashion, eating habits, new technology. On the other hand, non-material culture is very difficult to change and accept. Knowledge, ideas, and beliefs are rooted in society and change in these aspects is not easily accepted by the society. A gap between material culture and non-material culture is known as Cultural Lag.
Question 2.
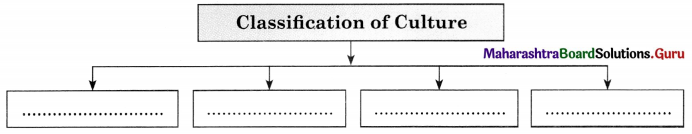
Classification of Culture.
Answer:
1. High Culture : High culture refers to cultural creations that have high status. For example, work of classical composers like Bhimsen Joshi, Hariprasad Chourasia, Ravi Shankar or the literature of Shakespeare.
2. Folk Culture: Folk culture refers to the culture of ordinary people, particularly those living in pre-industrial societies. For example, folk music, folk tales which are handed down from generation to generation. Bhangada in Punjab and Lavani in Maharashtra.
3. Mass Culture : Mass culture is a product of industrial societies and essentially a product of mass media. For example, popular feature films, TV soap-operas and recorded pop music.
4. Popular Culture : Popular culture includes any cultural product appreciated by a large number of ordinary people with no great cultural expertise. For example, mass market films such as Star Wars, Titanic, Harry Potter books and Chhota Bhim etc.
5. Subculture : Subcultures refer to groups of people that have something in common with each other which distinguishes them from other social groups. For example, culture shared by religious or ethnic groups.
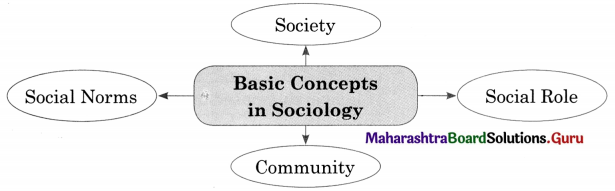
Question 3.
Components of Culture.
Answer:
1. Symbols : Culture is a system of symbols. Every culture is filled with symbols that signifies something and often evoke various reactions and emotions. People who share a culture often attach a specific meaning to an object, sound or image. For example, with the extensive use of mobile, emoji’s emotions are widely used to express and communicate various emotions.
2. Language : Language is a set of socially sound patterns, words and sentences having specific meaning and terminology common to the same culture. Language is a source of communication to transmit messages from one person to another. Language is like a vehicle through which we can carry out our complex social activities.
3. Knowledge : With the help of knowledge an individual knows how to cope with the existing social situation. It is one of the most important elements of culture. Knowledge could be direct or indirect. Direct knowledge is immediate knowledge whereas indirect knowledge is mediated knowledge.
4. Values and Beliefs : Values involves standards of what is good or bad, and desirable or undesirable. Values depend upon culture. Some values are hereditary which we gain from our elders and parents. Culture is full of values which are transmitted from one generation to another. Beliefs are statements that people hold to be true. While beliefs are specific, particular matters that individual consider to be true or false, values are abstract standards of goodness.
5. Norms : Norms are rules and behavioural expectations by which a society guides the behaviours of its members. Norms tell us how we should believe in specific situation. Some norms are prospective and some are perspective norms.
Explain the following concept with suitable examples.
Question 1.
Cultural Lag
Answer:
- The material aspect of culture changes very fast. It is convenient to adapt to new fashion, eating habits, new technology.
- On the other hand, non-material culture which is abstract, is very difficult to change. Knowledge, ideas and beliefs are rooted in society for many decades and centuries. Change in these aspects is not readily accepted by society.
- Over a period of time there is a gap between material and non-material culture. This gap is known as cultural lag.
Example : Medical technology has advanced at such a pace so as to put it in conflict with several moral and ethical beliefs.
![]()
Question 2.
Symbols
Answer:
- A symbol is anything that is used to stand for something else. People who share a culture often attach a specific meaning to an object, gesture, sound or image.
- Every culture is filled with symbols, or things that stand for something else and that often evoke various reactions and emotions.
- The first element that exists in every culture is a variety of symbols. Culture is a system of symbols.
Example : Use of mobile emoji’s emotions to express and communicate various emotions. The Indian flag represents our entire country. An amber light at a traffic intersection is used to convey the message that one can be ready to stop/start one’s vehicle.
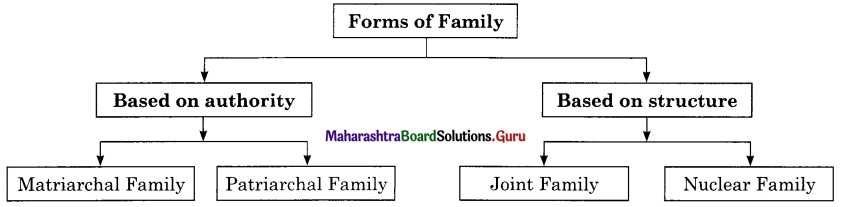
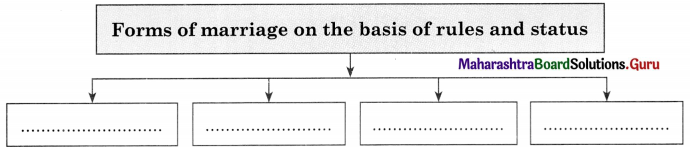
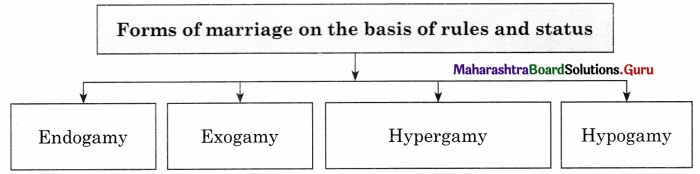
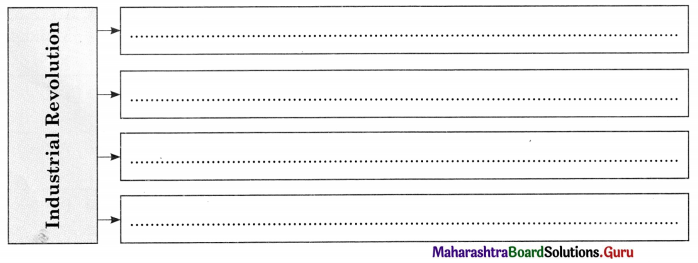
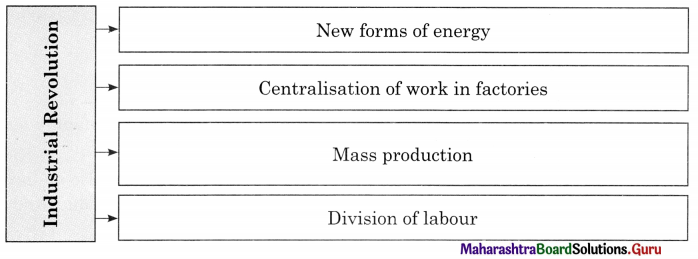
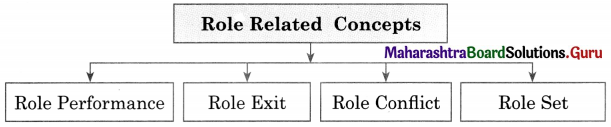
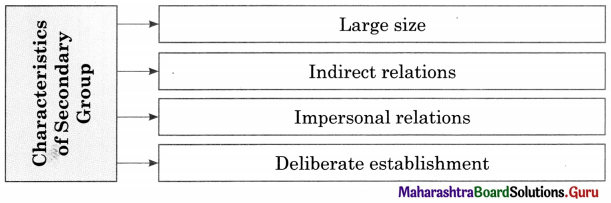
Complete the concept maps.
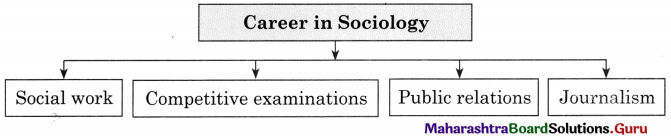
Question 1.
Answer:
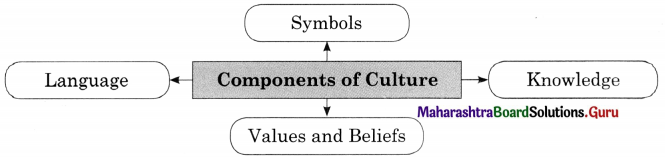
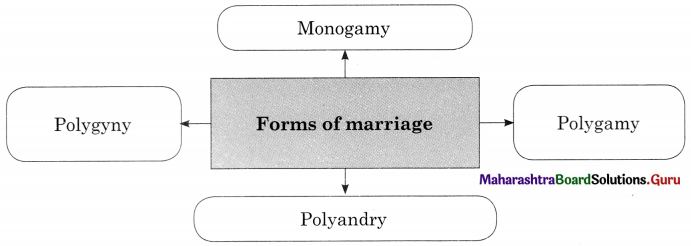
Question 2.
Answer:
Question 3.
Answer:

Question 4.

Answer:

![]()
Question 5.
Answer:
Question 6.

Answer:

Question 7.
Answer:

State whether the following statements are true or false with reasons.
Question 1.
Folkways are strictly held beliefs about behaviours.
Answer:
This statement is False.
- Folkways are mildly enforced social expectations.
- Folkways are customary normal and habitual ways of a group to meet certain needs or to solve day to day problems and don’t have serious binding on groups.
- The manner of speech; dressing; forms of etiquette and numerous other practices of daily life are some of examples of customary practices to which individuals conform in their personal habits. They have very serious binding on groups.
Question 2.
Culture is man-made.
Answer:
This statement is True.
- Culture is not a force, operating by itself but it’s a creation of society through interaction.
- Culture is a creation of society through interaction and depends for its existence upon the continuance of society. Culture, in short, is a human product; it is not natural.
- Culture consists of the intellectual, artistic and social ideals and institutions which the members of the society profess and to which they strive to conform. Hence culture is man made.
![]()
Question 3.
Culture is stagnant.
Answer:
This statement is False.
- Culture is continually changing : With the passing of time, some beliefs change, certain traditions or rituals are eliminated, language and mannerisms of people change, and thus, their culture.
- Migration and globalisation leads to contribute to the formation of a multicultural society and sometimes, even new cultures develop.
- Due to education and increased awareness, newer generations become flexible to change So some rituals or customs become less rigid or some are discontinued. Hence all cultures change in time
Question 4.
Ethnocentrism has positive as well as negative side.
Answer:
This statement is True.
Ethnocentrism has positive as well as negative side according to many scholars-
- According to conflict theorists, ethnocentrism denies equal opportunities. On the other hand functionalist sociologists, claim that ethnocentrism serves to maintain a sense of solidarity.
- The negative side is that the ethnocentrism can lead to arrogance towards other culture leading to a biased understanding of the same.
- The positive side is that it offers confidence to the culture thus helping a group remain cohesive and centred.
Question 5.
Cultural hybridisation is delayed through globalisation.
Answer:
This statement is False.
Cultural hybridisation is sped up through globalisation.
- Easy flow of migration, information, goods and services and exchanges of traditions has made the hybridisation process quick.
- In this process, two originally distinct cultures come together and create something new and exciting.
- We see hybridisation in many aspects of culture like food, language, wedding practices, dressing.
Give your personal response.
Question 1.
Emoji’s emotions are widely used to express and communicate various emotions.
Answer:
Emojis emotions are an example of a symbol which is anything that is used to stand for something
else. Emojis / Smileys are combinations of keyboard characters that may use to represent their feelings online or through texting. They are early identified thought or feeling and help us to add clarity to our communication.
Question 2.
Indian woman owns much to the western in one direction, she is still much the same as she was in another.
Answer:
This is an example of cultural lag where the material aspect, as compared with the non-material tends to progress rapidly. It is not true that there has been no transformation but it is the fact that the change is very little when compared with the change that has occurred in other directions.
Question 3.
Creativity and cultural engagement have shown to improve both mental and physical health.
Answer:
Participation in culture contributes to a healthy population in several ways. Inter cultural understanding and identity formation can be promoted by participation in arts which can further relieve isolation. It gives a common ground for people to assimilate.
![]()
Answer the following in detail (About 150 words).
Question 1.
In contemporary society global styles are given a unique local flavour. Explain the concept of cultural hybridisation with examples.
Answer:
Cultural hybridisation refers to the ways in which parts of one culture get recombined with the cultures of another. In the globalisation process, some forms of new and different cultural practice or behaviour develops from the mixing of different cultural traditions. The groups do not necessarily give up their own culture but participate in various ways in each other’s cultural activities.
Easy flow of migration, information, goods and services and exchanges of traditions has made the hybridisation process quick. In this process, two originally distinct cultures come together and create something new and exciting. We see hybridisation in many aspects of culture like food, language, wedding practices, dressing habits so on and so forth. Today we prefer Italian pizza with tandoori paneer as topping which indeed is very Indian. Celebration of Valentine’s Day, language mixing, fusion music are examples of cultural hybridisation.
In order to understand cultural hybridisation, one needs to understand the process of glocalisation. Global processes interact with local processes. In contemporary society, global styles are given a unique local flavour, e.g. A global restaurant chain modifying their menus based on the unique culture they are in but maintaining their brand. For example, Indian spicy taste is incorporated in the otherwise differently flavoured burger of McDonald’s or Indianization of Chinese food.