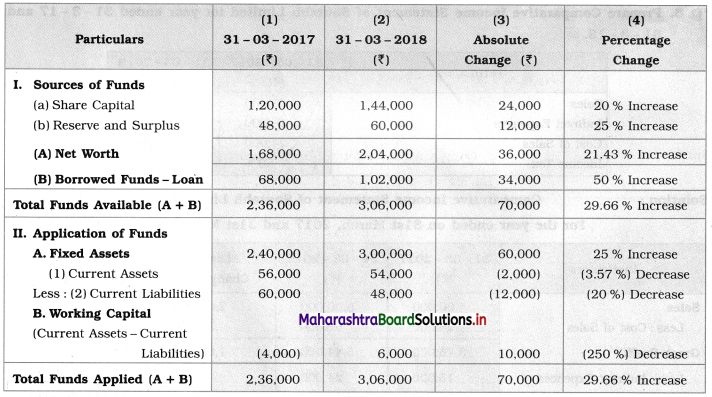
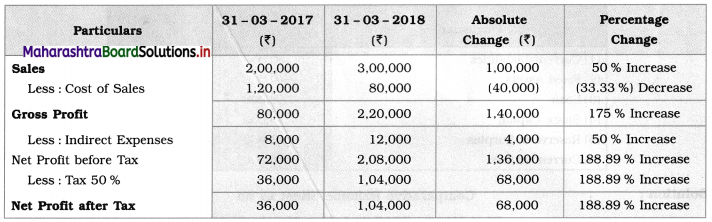
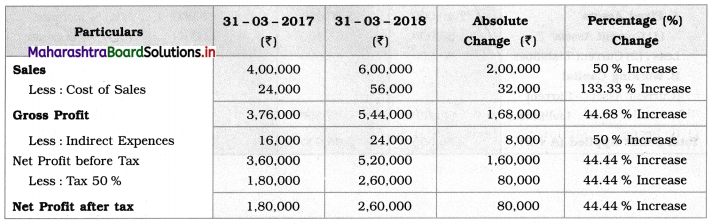
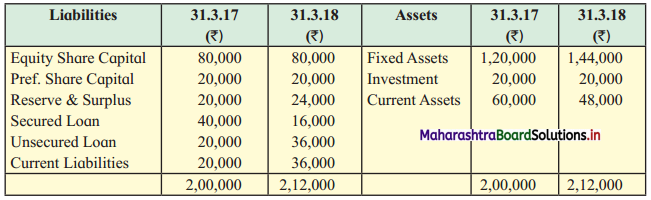
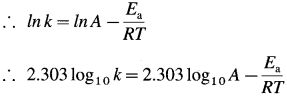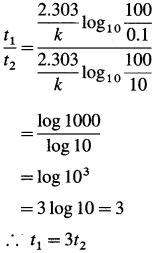Balbharti Maharashtra State Board 12th Chemistry Textbook Solutions Chapter 13 Amines Textbook Exercise Questions and Answers.
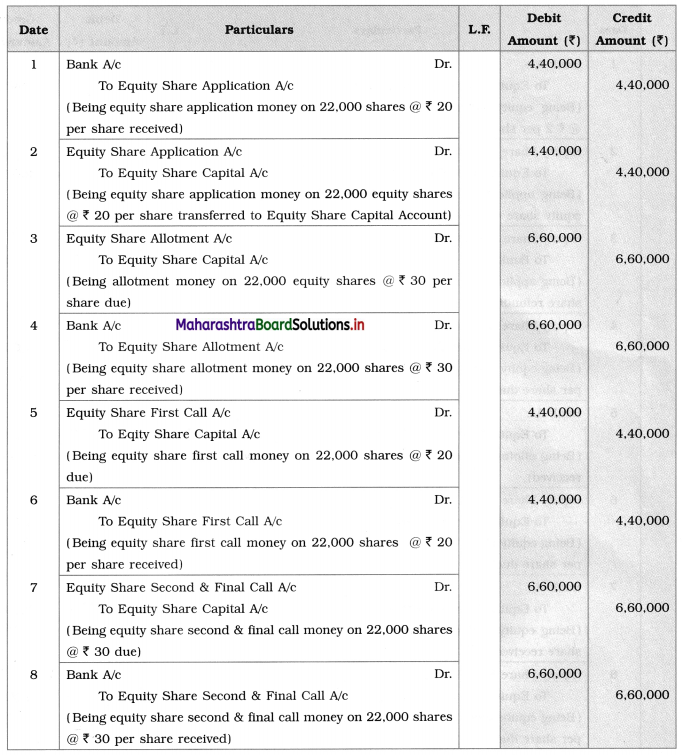
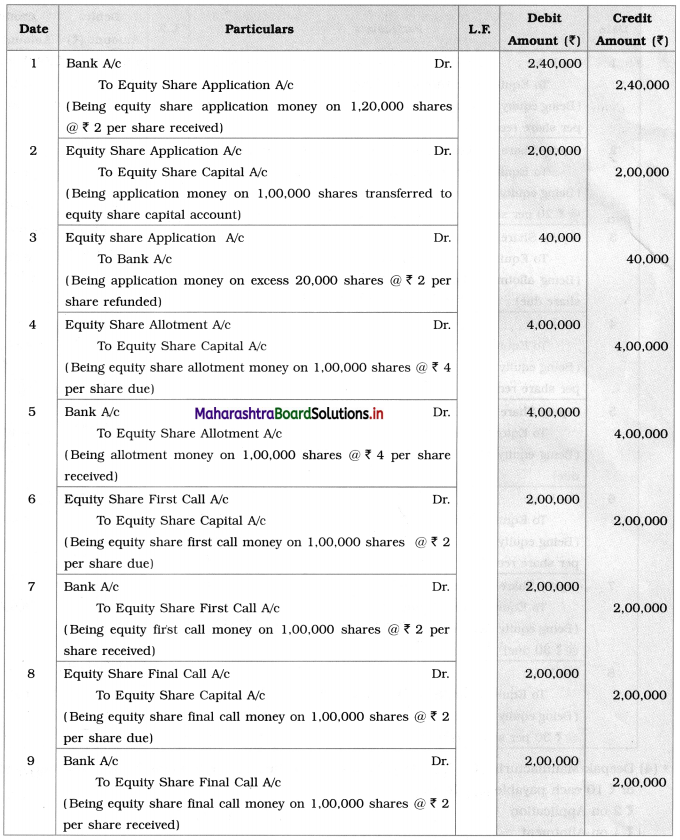
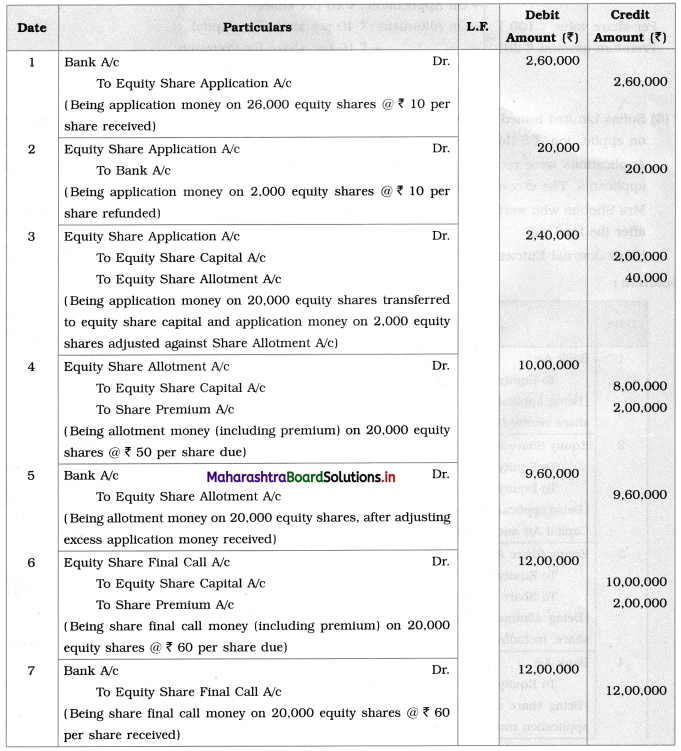
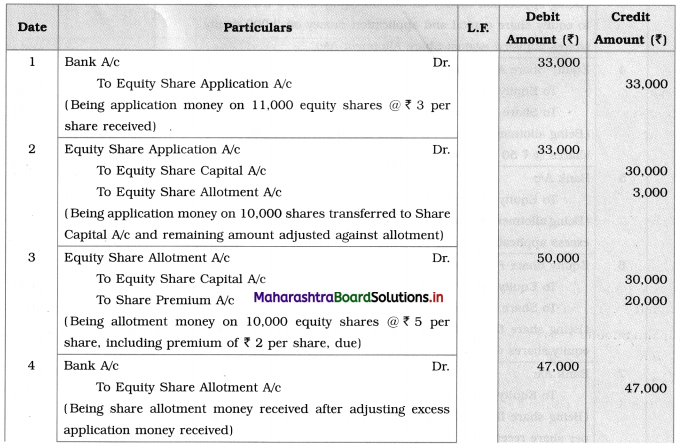
Maharashtra State Board Class 12 Chemistry Solutions Chapter 13 Amines
1. Choose the most correct option.
Question i.
The hybridization of nitrogen in primary amine is ………………………..
a. sp
b. sp2
c. sp3
d. sp3d
Answer:
c. sp3
![]()
Question ii.
Isobutylamine is an example of ………………………..
a. 2° amine
b. 3° amine
c. 1° amine
d. quaternary ammonium salt.
Answer:
a. 2° amine
Question iii.
Which one of the following compounds has the highest boiling point?
a. n-Butylamine
b. sec-Butylamine
c. isobutylamine
d. tert-Butylamine
Answer:
a. n-Butylamine
Question iv.
Which of the following has the highest basic strength?
a. Trimethylamine
b. Methylamine
c. Ammonia
d. Dimethylamine
Answer:
d. Dimethylamine
Question v.
Which type of amine does produce N2 when treated with HNO2?
a. Primary amine
b. Secondary amine
c. Tertiary amine
d. Both primary and secondary amines
Answer:
a. Primary amine
Question vi.
Carbylamine test is given by
a. Primary amine
b. Secondary amine
c. Tertiary amine
d. Both secondary and tertiary amines
Answer:
a. Primary amine
Question vii.
Which one of the following compounds does not react with acetyl chloride?
a. CH3-CH2-NH2
b. (CH3-CH2)2NH
c. (CH3-CH2)3N
d. C6H5-NH2
Answer:
Answer:
c. (CH3 – CH2)3N
Question viii.
Which of the following compounds will dissolve in aqueous NaOH after undergoing reaction with Hinsberg reagent?
a. Ethylamine
b. Triethylamine
c. Trimethylamine
d. Diethylamine
Answer:
a. Ethyl amine
![]()
Question ix.
Identify ‘B’ in the following reactions
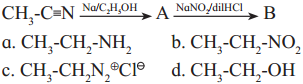
Answer:
d. CH3-CH2-OH
Question x.
Which one of the following compounds contains azo linkage?
a. Hydrazine
b. p-Hydroxyazobenzene
c. N-Nitrosodiethylamine
d. Ethylenediamine
Answer:
b. p-Hydroxyazobenzene
2. Answer in one sentence.
Question i.
Write reaction of p-toluenesulfonyl chloride with diethylamine.
Answer:

Question ii.
How many moles of methylbromide are required to convert ethanamine to N, N-dimethyl ethanamine?
Answer:
2 moles of methylbromide are required to convert ethanamine to N, N-dimethyl ethanamine.
Question iii.
Which amide does produce ethanamine by Hofmann bromamide degradation reaction?
Answer:
Propanamide (CH3 – CH2 – CONH2) produces ethanamine by Hofmann bromamide degradation reaction.
Question iv.
Write the order of basicity of aliphatic alkylamine in gaseous phase.
Answer:
The order of basicity of aliphatic alkyl amines in the gaseous follows the order : tertiary amine > secondary amine > primary amine > NH3.
Question v.
Why are primary aliphatic amines stronger bases than ammonia?
Answer:
The alkyl group tends to increase the electron density on the nitrogen atom. As a result, amines can donate the lone f pair of electrons on nitrogen more easily than ammonia. Hence, aliphatic amines are stronger bases than ammonia.
Question vi.
Predict the product of the following reaction. Nitrobenzene Sn/Conc. HCl?
Answer:
The product is aniline/
Question vii.
Write the IUPAC name of benzylamine.
Answer:
The IUPAC name is Phenylmethanamine.
Question viii.
Arrange the following amines in an increasing order of boiling points. n-propylamine, ethylmethyl amine, trimethylamine.
Answer:
Amines in an increasing order of boiling points : trimethyl amine, ethyl methyl amine, n-propyl amine
Question ix.
Write the balanced chemical equations for the action of dil H2SO4 on diethylamine.
Answer:

![]()
Question x.
Arrange the following amines in the increasing order of their pKb values. Aniline, Cyclohexylamine, 4-Nitroaniline
Answer:
Cyclohexyl amine (pKA 3.34), aniline (pKA 9.13) 4-nitroaniline (pKA 12.99)
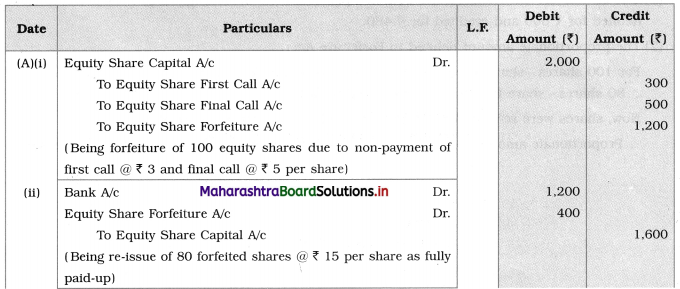
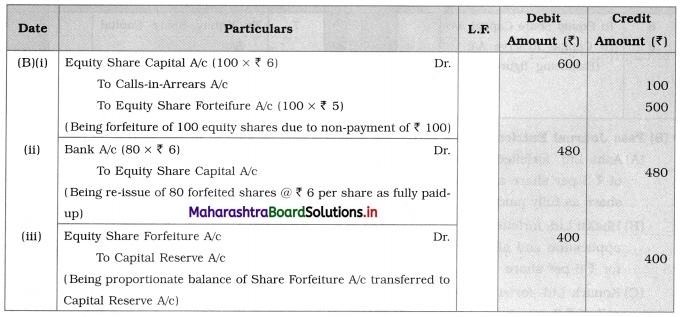
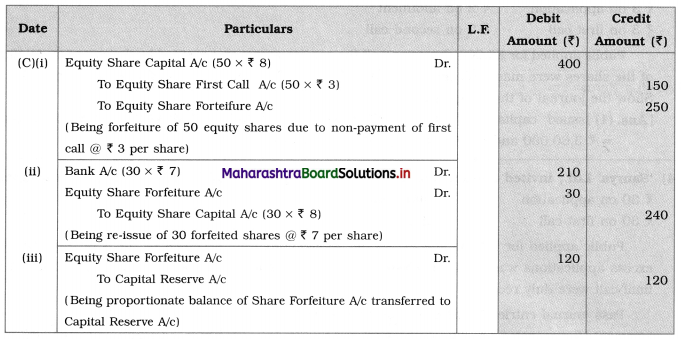
3. Answer the following
Question i.
Identify A and B in the following reactions.
![]()
Answer:
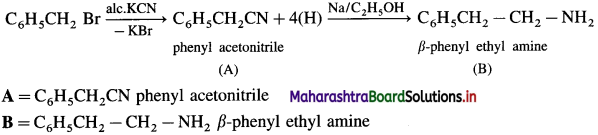
Question ii.
Explain the basic nature of amines with suitable example.
Answer:
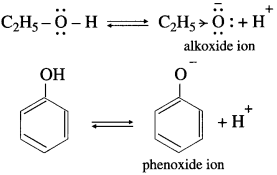
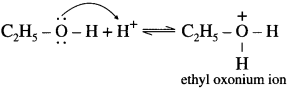
The basic strength of amines is expressed in terms of Kb or pKb value. According to Lowry-Bron-sted theory the basic nature of amines is explained by the following equilibrium equation.
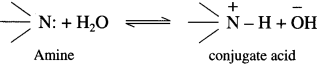
In this equilibrium amine accepts H+, hence an amine is a Lowry-Bronsted base.
According to Lewis theory, the species which donates a pair of electrons is called a base.
The nitrogen atom in amiqes has a lone pair of electrons, which can be donated to suitable acceptor like proton H+.
The aqueous solutions of amines are basic in nature due to release of free OH– ions in solutions. Hence amines are Lewis bases. There exists an equilibrium in their aqueous solutions as follows :
R – NH2 + H2O ⇌ RNH3 + OH–
Since OH– is a stronger base, equilibrium shifts towards left-hand side giving less concentration of OH–.
Here, Kb value is smaller and pKb value is larger.
Hence amines are weak bases.
Question iii.
What is diazotisation? Write diazotisation reaction of aniline.
Answer:
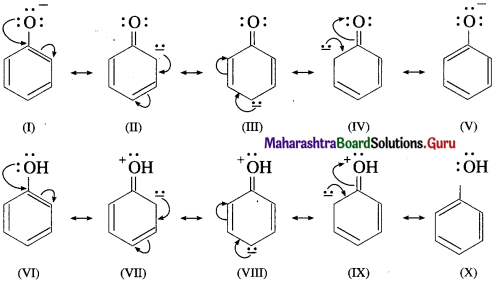
Aryl amines react with nitrous acid in cold condition (273 – 278 K) forms arene diazonium salts. The conversion of primary aromatic amine into diazonium salts is called diazotisation.
Diazotisation of aniline :

Question iv.
Write reaction to convert acetic acid into methylamine.
Answer:

Question v.
Write a short note on coupling reactions.
Answer:
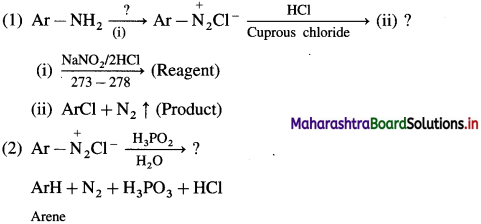
Reactions involving retention of diazo group : (Coupling reactions) :
![]()
Question vi.
Explain Gabriel phthalimide synthesis.
Answer:
Phthalimide is reacted with alcoholic KOH to form potassium phthalimide. Further potassium phthalimide is treated with an ethyl iodide. The product N-ethylphthalimide is hydrolysed with aq NaOH to form ethyl amine. This reaction is known Gabriel phthalimide synthesis.
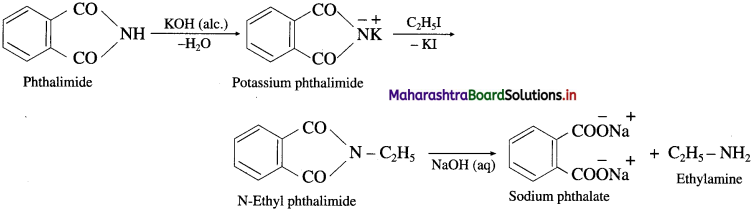
Question vii.
Explain carbylamine reaction with suitable examples.
Answer:
Aliphatic or aromatic primary amines on heating with chloroform and alcoholic potassium hydroxide solution form carbyl amines or isocyanides with extremely unpleasant smell. This reaction is a test for primary amines.
Secondary and tertiary amines do not give this test.
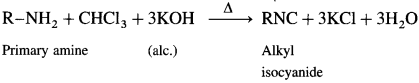

Question viii.
Write reaction to convert
(i) methanamine into ethanamine
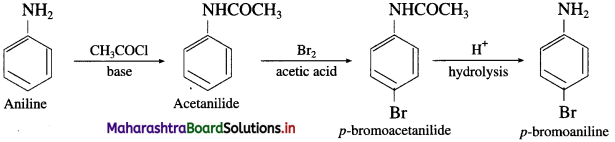
(ii) Aniline into p-bromoaniline.
Answer:
(1) Methanamine into ethanamine
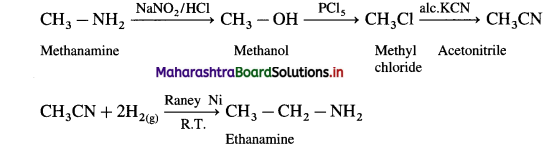
(2) Aniline into p-bromo aniline
Question ix.
Complete the following reactions :
a. C6H5N2 Cl + C2H5OH →
b. C6H5NH2 + Br2(aq) → ?
Answer:
(a)
![]()
(b)
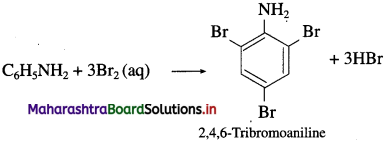
![]()
Question x.
Explain Ammonolysis of alkyl halides.
Answer:
When an alkyl halide is heated with alcoholic ammonia in a sealed tube under pressure at 373 K, a mixture of primary, secondary, tertiary amines and a quaternary ammonium salt is obtained. In this reaction, breaking of C – X bond by ammonia is called ammonolysis of alkyl halides. The reaction is also known as alkylation. For example, when methyl bromide is heated with alcoholic ammonia at 373 K, it gives a mixture of methylamine (a primary amine), dimethylamine (a secondary amine), trimethyl amine (a tertiary amine) and tetramethylam- monium bromide (a quaternary ammonium salt).
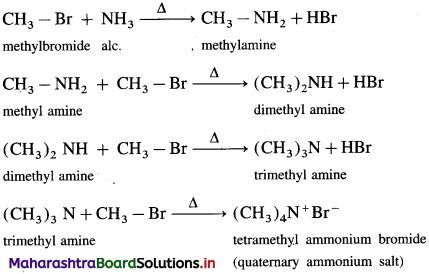
The order of reactivity of alkyl halides with ammonia is R – I > R – Br > R – Cl.
Question xi.
Write reaction to convert ethylamine into methylamine.
Answer:

4. Answer the following.
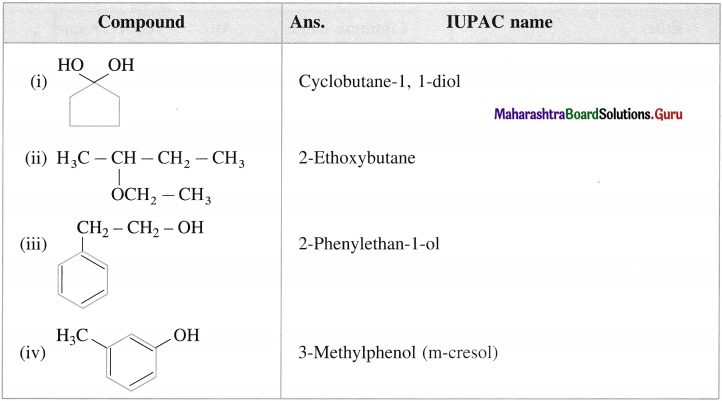
Question i.
Write the IUPAC names of the following amines :
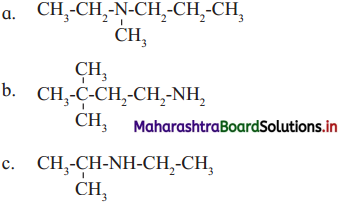
Answer:
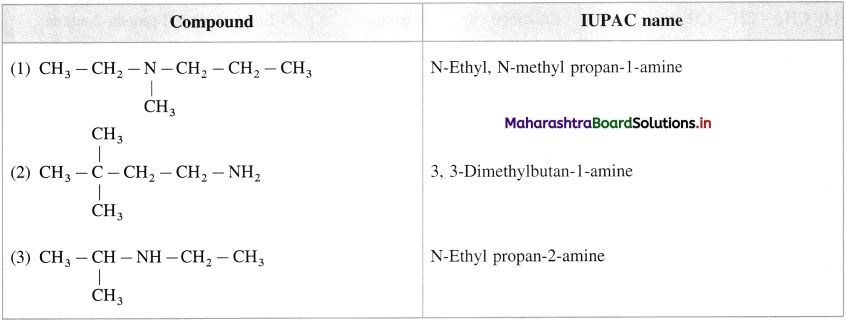
Question ii.
What are amines? How are they classified?
Answer:
Amines are classified on the basis of the number of hydrogen atoms of ammonia that are replaced by alkyl group. Amines are classified as primary (1°), secondary (2°) and tertiary (3°).
(1) Primary amines (1° amines) : The amines in which only one hydrogen atom of ammonia is replaced by an alkyl group or aryl group are called primary (1°) amines.
Examples :
(i) CH3 – NH2 methylamine
(ii) CH3 – CH2 – NH2 ethylamine

(2) Secondary amines (2° amines) : The amines in which two hydrogen atoms of ammonia are replaced by two, same or different alkyl or aryl groups are called secondary (2°) amines.
Examples :
(i) C2H5 – NH – CH3 ethylmethylamine
(ii) CH3 – NH – CH3 dimethylamine
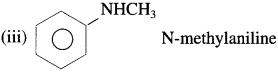
![]()
(3) Tertiary amines (3° amines) : The amines in which all the three hydrogen atoms of ammonia are replaced by three same or different alkyl or aryl groups are called tertiary (3°) amines.
Examples :
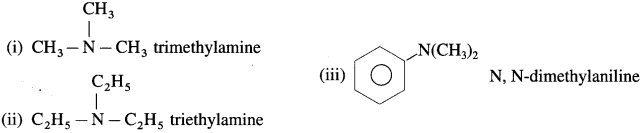
Secondary and tertiary amines are further classified as (1) Simple or symmetrical amines (2) Mixed or unsymmetrical amines.
(i) Simple or symmetrical amines : In simple amines same alkyl groups are attached to the nitrogen e.g.

(ii) Mixed or unsymmetrical amines : In mixed amines different alkyl groups are attached to the nitrogen.
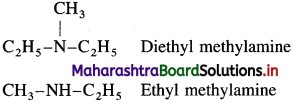
Question iii.
Write IUPAC names of the following amines.
Answer:

Question iv.
Write reactions to prepare ethanamine from
a. Acetonitrile
b. Nitroethane
c. Propionamide
Answer:
a. Ethanamine from acetonitrile :
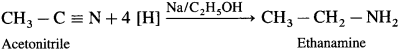
b. Ethanamine from nitroethane :
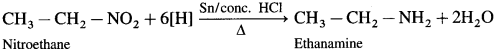
c. Ethanamine from Propionamide :
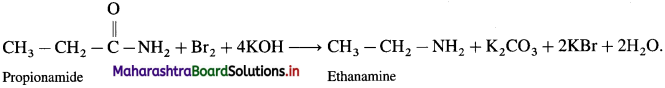
![]()
Question v.
What is the action of acetic anhydride on ethylamine, diethylamine and triethylamine?
Answer:
Acetylation of amines : The reaction in which the H atom attached to nitrogen in amine is replaced by acetyl group 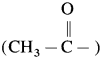 is called acetylation of amines.
is called acetylation of amines.
(1) Ethylamine on reaction with acetic anhydride forms monoacetyl derivative, N-acetylethylamine.

(2) Diethylamine (a secondary amine) on reaction with acetic anhydride forms a monoacetyl derivative, N-acetyldiethyl amine (or N,N-diethyl acetamide).

(3) Triethylamine does not react with acetic anhydride as it does not have any H atom attached nitrogen atom of amin e
![]()
Question vii.
Distinguish between ethylamine, diethylamine and triethylamine by using Hinsberg’s reagent?
Answer:
This reaction is useful for the distinction of primary, secondary and tertiary amines.
(i) Primary amine (like ethyl amine) is treated with Hinsberg’s reagent (benzene sulphonyl chloride) forms N-alkyl benzene sulphonamide which dissolve in aqueous KOH solution to form a clear solution of potassium salt and upon acidification gives insoluble N-alkyl benzene sulphonamide.

(ii) Secondary amine like diethyl amine is treated with benzene sulphonyl chloride forms N,N-diethyl benzene which sulphonyl amide remains insoluble in aqueous KOH and does not dissolve in acid.

(iii) Tertiary amine like triethyl amine does not react with benzene sulphonyl chloride and remains insoluble in KOH, however it dissolves in dil. HCl to give a clear solution due to formation of ammonium salt.
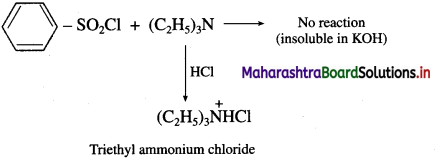
![]()
Question viii.
Write reactions to bring about the following conversions :
a. Aniline into p-nitroaniline
b. Aniline into sulphanilic acid?
Answer:
(1) Aniline into p-nitroaniline
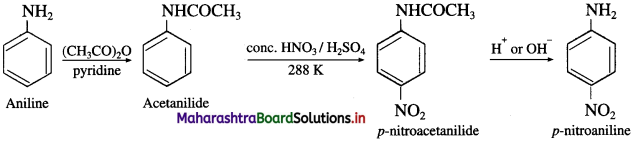
(2) Aniline into sulphanilic acid.
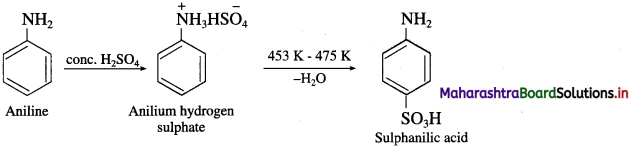
Activity :
- Prepare a chart of azodyes, colours and its application.
- Prepare a list of names and structures of N-containing ingredients of diet.
12th Chemistry Digest Chapter 13 Amines Intext Questions and Answers
Use your brain power! (Textbook Page No 282)
Question 1.
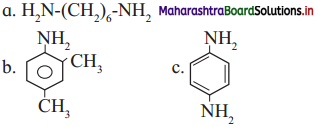
Classify the following amines as simple/mixed; 1°, 2°, 3° and aliphatic or aromatic. (C2H5)2NH, (CH3)3N, C2H5 – NH – CH3,

Answer:

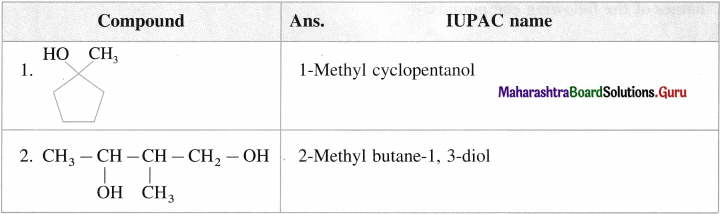
(A) Common Names : Rules
- According to common naming system, the amines are named as alkylamines.
- The common name of a primary amine is obtained by writing the name of the alkyl group followed by the word ‘amine’.
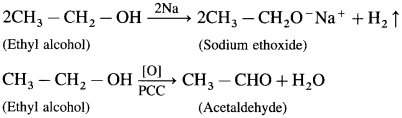
Example : CH3 – NH2 : methyl-amine - The simple {symmetrical) secondary and tertiary amines are written by adding prefix ‘di- (forpresence of two alkyl groups) and ‘tri’- (for presence of three alkyl groups) respectively to the name of alkyl groups.
Examples: (i) CH3 – NH – CH3 dimethylamine, (ii) (C2H5)3 N triethylamine - The mixed (or unsymmetrical) secondary and tertiary amines are given names by writing the names of alkyl groups in alphabetical order, followed by the word ‘amine’.
Example : CH3 – CH2 – NH – CH3 ethyhnethylamine
![]()
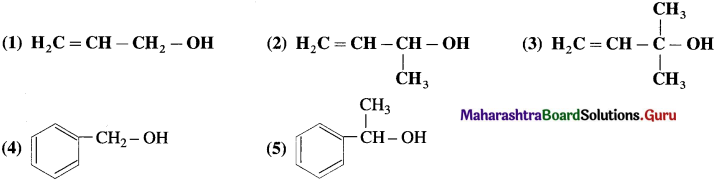
(B) IUPAC names : Rules
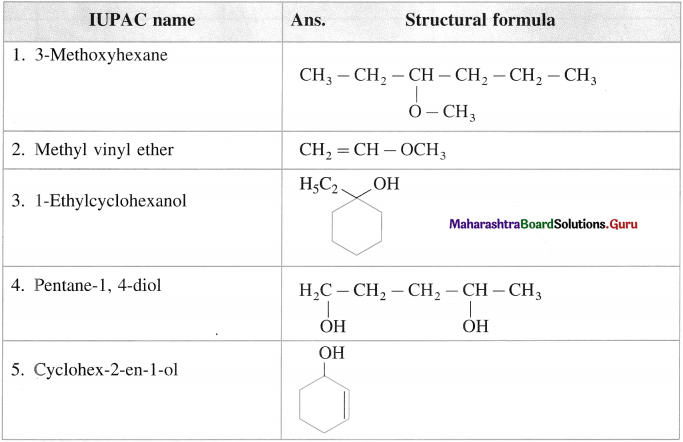
- According to IUPAC system of nomenclature of amines, aliphatic amines are named as alkanarnines.
- The name of the amine is obtained by replacing the suffix ‘e’ from parent alkane’s name by ‘amine’.
- The position of the amino group is indicated by the lowest possible locant.
Example :
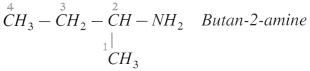
- In case of secondary and tertiary amines, the largest alkyl group is considered to be the parent alkane and other alkyl groups are written as N-substituents.
Example : ClH5NH – CH3 N – Methylethanamine - A complete name of amine is written as one word.
Try this….. (Textbook Page No 283)
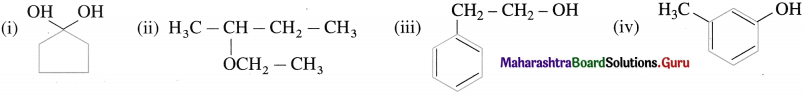
Question 1.
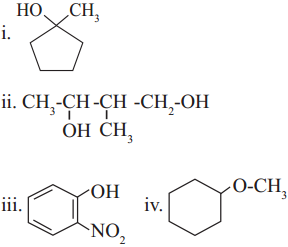
Draw possible structures of all the isomers of C4H11N. Write their common as well as IUPAC names.
Answer:


Use your brain power! (Textbook Page No 283)
Question 1.
Write chemical equations for
(i) reaction of alc. NH with C2H5I.
Answer:

(ii) Amonolysis of benzyl chloride followed by the reaction with 2 moles of CH3I.
Answer:

(2) Ammonolysis of alkyl halides is not suitable method to prepare primary amines.
Answer:
In the laboratory, ammonolysis of alkyl halides is not a suitable method to prepare primary amines as it gives a mixture of primary, secondary, tertiary amines and quaternary ammonium salts. (Refer to the reaction in answer to Question 16). The separation of primary amine becomes difficult.
![]()
Problem 13.1 : (Textbook Page No 285)
Question 1.
Write reaction to convert methyl bromide into ethyl amine? Also, comment on the number of carbon atoms in the starting compound and the product.
Solution :
Methyl bromide can be converted into ethyl amine in two stage reaction sequence as shown below.
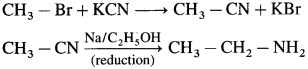
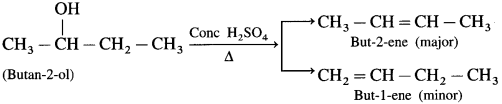
The starting compound methyl bromide contains one carbon atom while the product ethylamine contains two carbon atoms. A reaction in which number of carbons increases involves a step up reaction. The overall conversion of methyl bromide into ethyl amine is a step up conversion.
Use your brain power! (Textbook Page No 285)
Identify ‘A’ and ‘B’ in the following conversions.
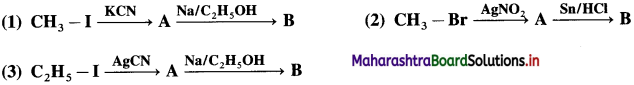
Answer:

Use your brain power! (Textbook Page No 286)
Question 1.
Write the chemical equations for the following conversions :
(1) Methyl chloride to ethylamine.
(2) Benzamide to aniline.
(3) 1, 4-Dichlorobutane to hexane-1, 6-diamine.
(4) Benzamide to benzylamine.
Answer:
(1) Methyl chloride to ethylamine
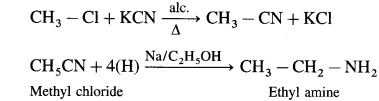
(2) Benzamide to aniline
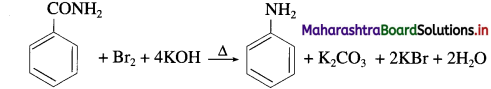
(3) 1, 4-Dichlorobutane to hexane-1, 6-diamine

(4) Benzamide to benzylamine
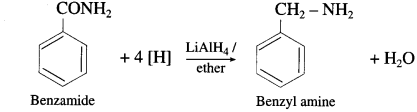
![]()
Use your brain power! (Textbook Page No 287)
Question 1.
Arrange the following :
(1) In decreasing order of the boiling point C2H5 – OH, C2H5 – NH2, (CH3)2 NH
(2) In increasing order of solubility in water: C2H5 – NH2, C3H7 – NH2, C6H5 – NH2
Answer:
(1) Decreasing order of the boiling point : C2H5 — OH, C2H5 — NH2, (CH3)2 NH
(2) Increasing order of solubility in water : C6H5NH2, C3H7 — NH2, C2H5 — NH2
Use your brain power! (Textbook Page No 288)
Question 1.
Refer to pKb values and answer which compound from the following pairs is the stronger base?
(1) CH3 – NH2 and (CH3)2 NH
(2) (C2H5)2 NH and (C2H5)3 N
(3) NH3 and (CH3)2 CH – NH2
Answer:
(1) CH3 -NH2 and (CH3)2 NH
(CH3)2 NH is a stronger base
(2) (C2H5)2 NH and (C2H5)3 N
(C2H5)2 NH is a stronger base
(3) NH3 and (CH3)2 CHNH2
(CH3)2 CHNH2 is a stronger base
Use your brain power! (Textbook Page No 290)
Question 1.
Arrange the following amines in decreasing order of their basic strength :
NH3, CH3 – NH2, (CH3)2 NH, C6H5NH2
Answer:
Decreasing order of basic strength :
(CH3)2NH, CH3 -NH2, NH3, C6H5NH2
Use your brain power! (Textbook Page No 291)
Question 1.

Answer:
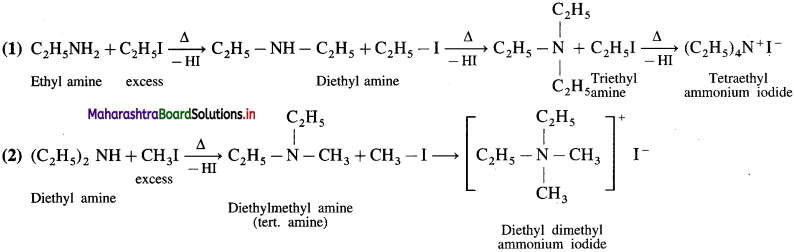
![]()
Use your brain power! (Textbook Page No 291)
Question 1.
Complete the following reaction :
![]()
Answer:

Use your brain power! (Textbook Page No 292)
![]()
Answer:

Use your brain power! (Textbook Page No 292)
Question 1.
Write the carbylamine reaction by using aniline as starting material.
Answer:

Can you tell? (Textbook Page No 292)
(1) What is the formula of nitrous acid ?
(2) Can nitrous acid be stored in bottle ?
Answer:
(1) Formula of nitrous acid : H – O – N = O
(2) Nitrous acid cannot be stored in bottle.
Use your brain power! (Textbook Page No 294)
Question 1.
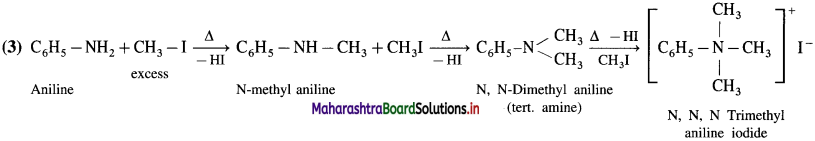
How will you distinguish between methyl amine, dimethylamine and trimethylamine by Hinsberg’s test?
Answer:
(1) Methyl amine (primary amine) reacts with benzene sulphonyl chloride to form N-methylbenzene sulphona- mide

(2) Dimethyl amine reacts with benzene sulphonyl chloride to give N, N – dimethylbenzene sulphonamide.

(3) Trimethyl amine does not react with benzene sulphonyl chloride and remains insoluble in KOH
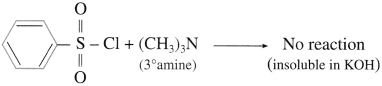
![]()
Problem 13.1 : (Textbook Page No 295)
Question 1.
Write the scheme for preparation of p-bromoaniline from aniline. Justify your answer.
Solution :
NH2 – group in aniline is highly ring activating and o – /p – directing due to involvement of the lone pair of electrons on ‘N’ in resonance with the ring. As a result, on reaction with Br2 it gives 2,4,6-tribromoaniline. To get a monobromo product, it is necessary to decrease the ring activating effect of – NH2 group. This is done by acetylation of aniline. The lone pair of ‘N’ in acetanilide is also involved in resonance in the acetyl group. To that extent, ring activation decreases.
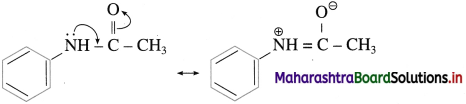
Hence, acetanilide on bromination gives a monobromo product p-bromoacetanilide. After monobromination the original – NH2 group is regenerated. The protection of – NH2 group in the form of acetyl group is removed by acid catalyzed hydrolysis to get p-bromoaniline, as shown in the following scheme.

Use your brain power! (Textbook Page No 296)
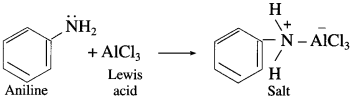
Question 1.
(1) Can aniline react with a Lewis acid?
(2) Why aniline does not undergo Frledel – Craft’s reaction using aluminium chloride?
Answer:
(1) Aniline reacts with a Lewis acid, forms salt.
(2) Aniline does not undergo Friedcl-Crafr’s reaction (alkylation and acetylation) due to salt formation with aluminium chloride (Lewis acid), which is used as catalyst. Due to this, nitrogen of anime acquires + ve charge and hence acts as strong deactivating effect on the ring and makes it difficult for electrophilic attack.
![]()
Can you tell? (Textbook Page No 294)
(1) Do tertiary amines have ‘H’ bonded to ‘N?
(2) Why do tertiary amines not react with benzene sulfonyl chloride?
Answer:
(1) Tertiary amines  do not have ‘H’ bonded to ‘N’.
do not have ‘H’ bonded to ‘N’.
(2) Tertiary amine does not undergo reaction with benzene sulphonyl chloride as it does not have any H atom attached to nitrogen atom of amine.
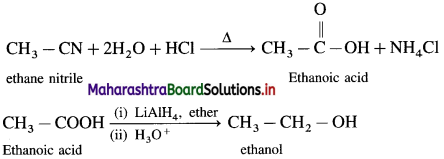

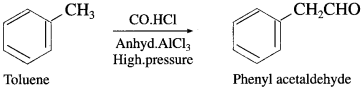
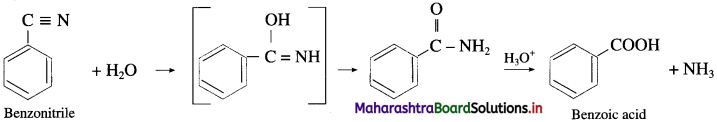
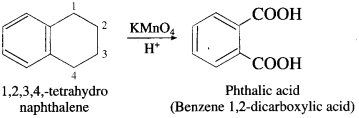

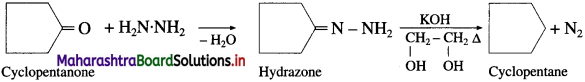

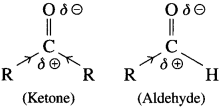
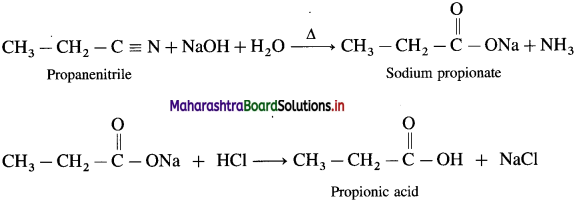

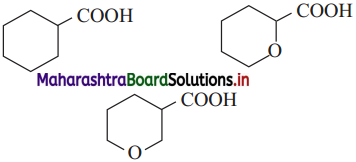
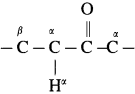
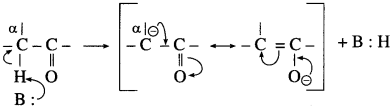
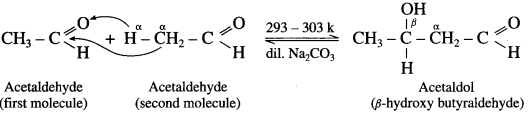
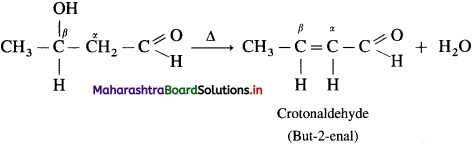
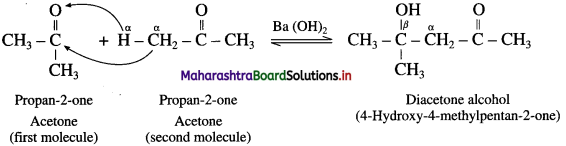

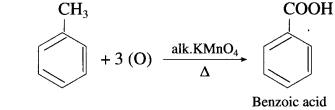


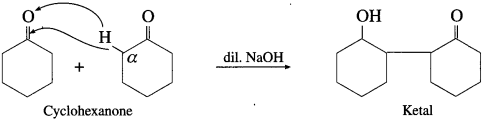
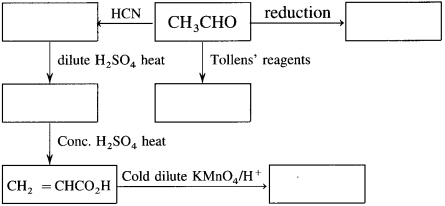



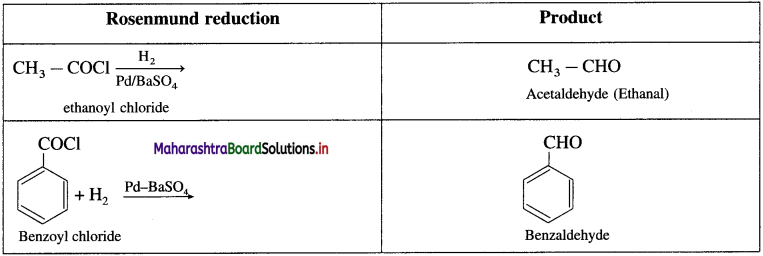
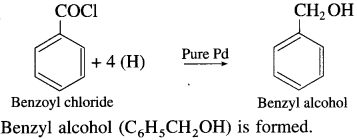
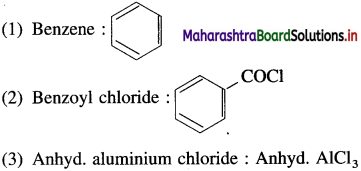
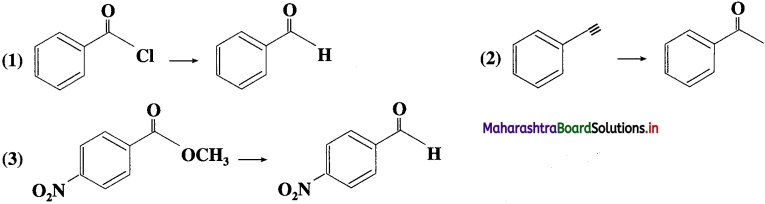
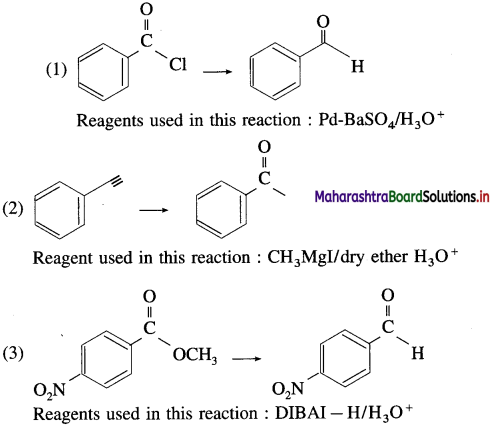
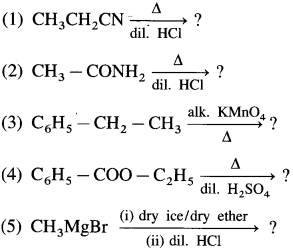
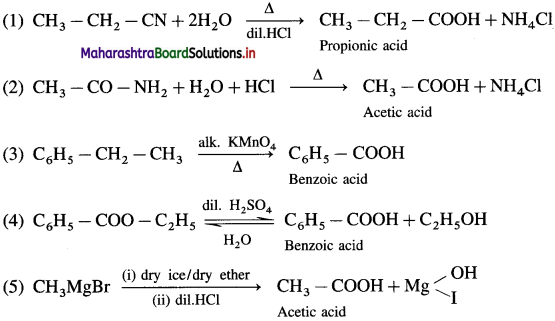

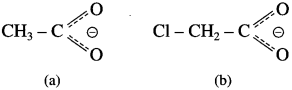
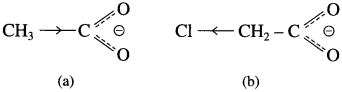
 The acetate ion formed gets destabilised due to electron releasing effect of methyl group
The acetate ion formed gets destabilised due to electron releasing effect of methyl group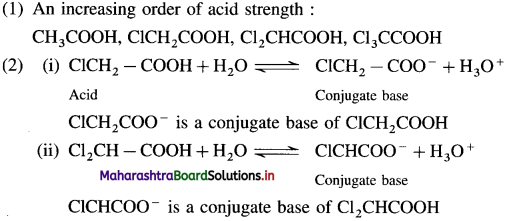
 The dichloroacetate ion formed gets stabilised due electron-withdrawing effect of two chlorine atoms. (- 1 effect)
The dichloroacetate ion formed gets stabilised due electron-withdrawing effect of two chlorine atoms. (- 1 effect)

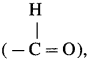 , an aldehyde is oxidised to carboxylic acid
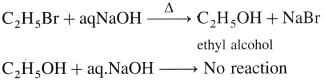
, an aldehyde is oxidised to carboxylic acid  which is not possible in case of ethers, ketones, alcohols and hydrocarbons.
which is not possible in case of ethers, ketones, alcohols and hydrocarbons. can’t be broken easily.
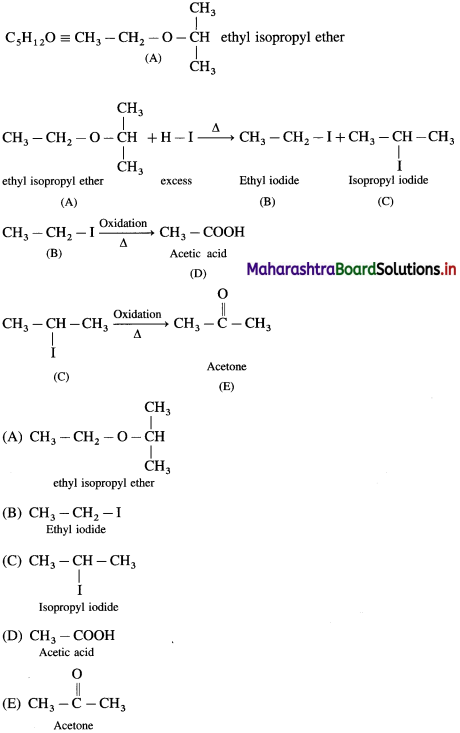
can’t be broken easily.
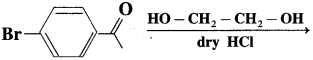
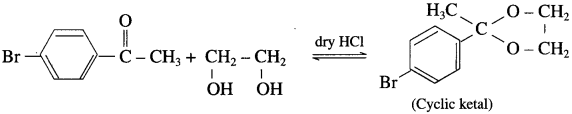


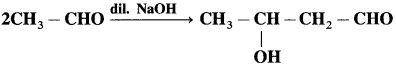
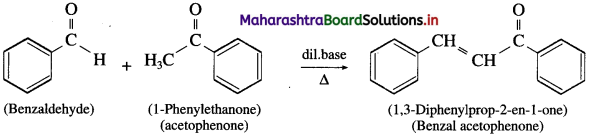
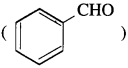 does not have a-hydrogen atom, it will not undergo self aldol condensation.
does not have a-hydrogen atom, it will not undergo self aldol condensation. contains a-carbon atom, it cannot undergo Cannizzaro reaction.
contains a-carbon atom, it cannot undergo Cannizzaro reaction.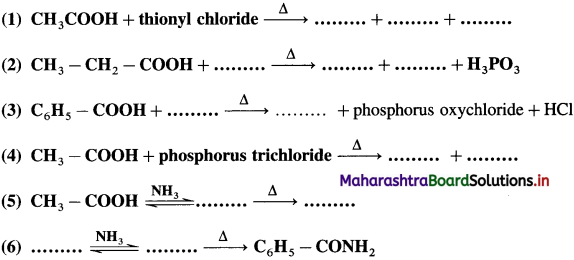






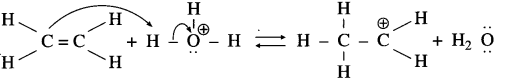
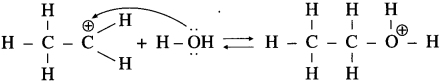
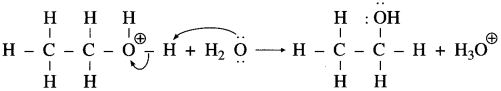

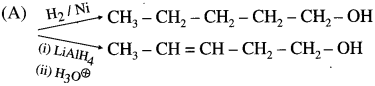
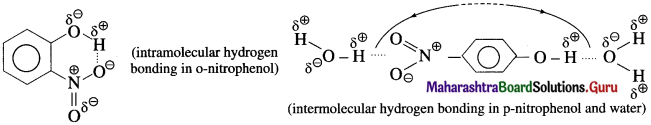


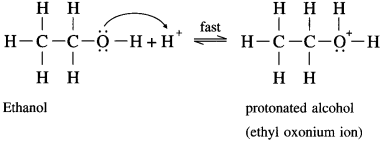
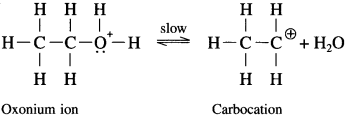
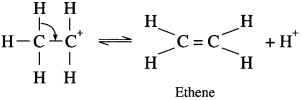
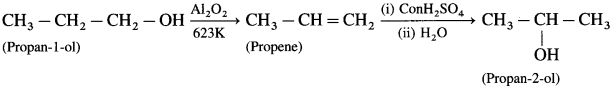
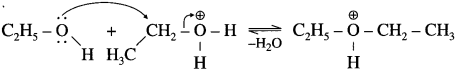


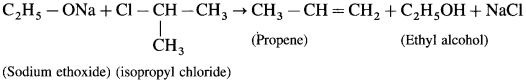
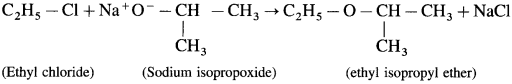
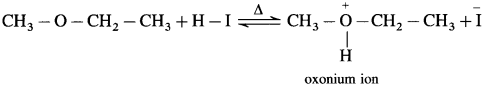
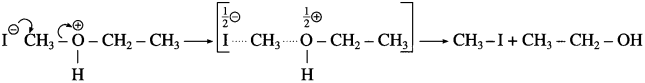
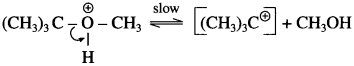
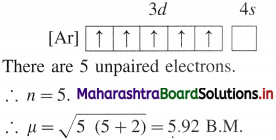
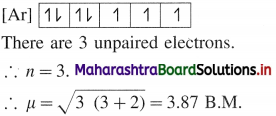
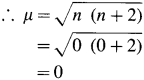
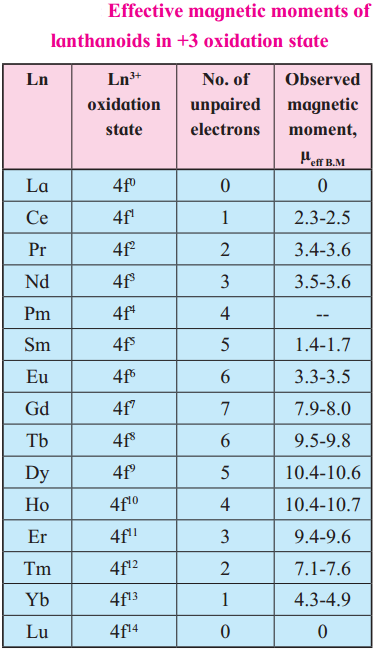






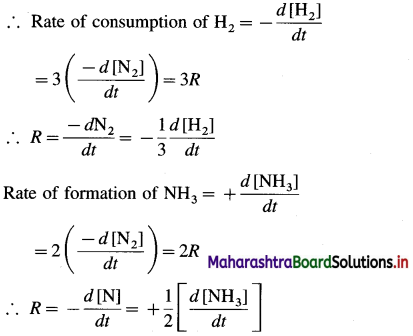
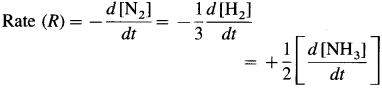

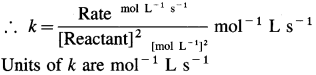
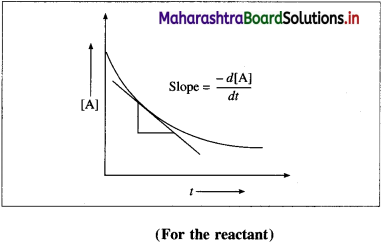
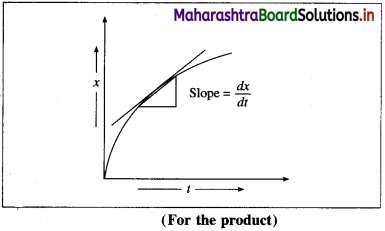
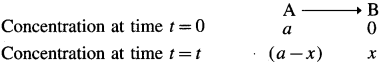
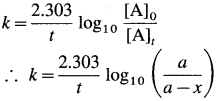

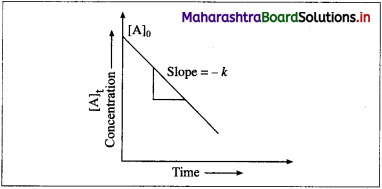

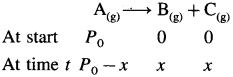
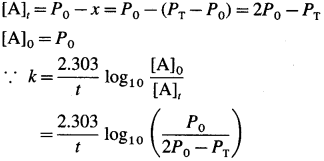

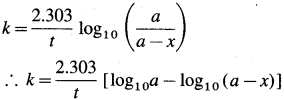


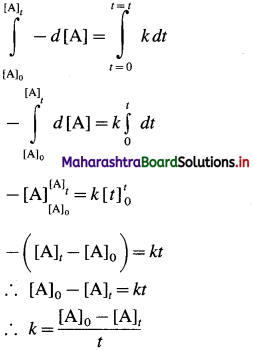
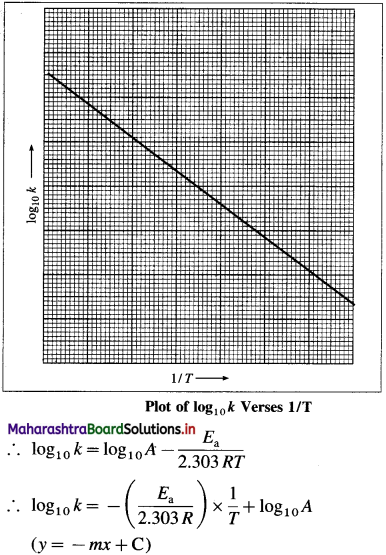
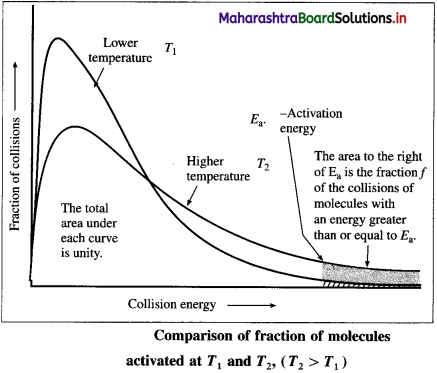
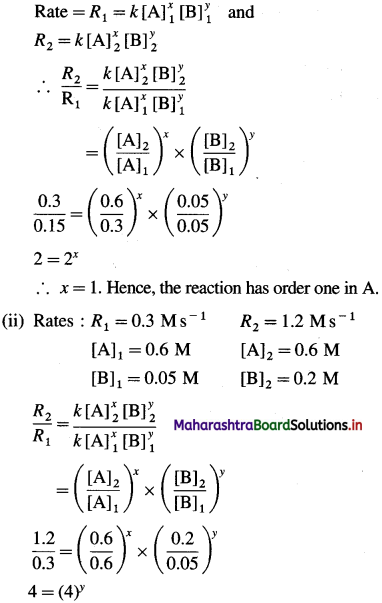
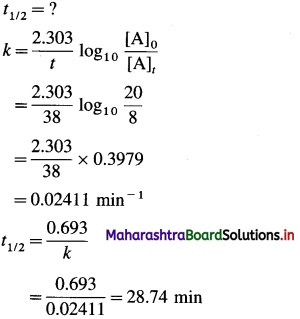
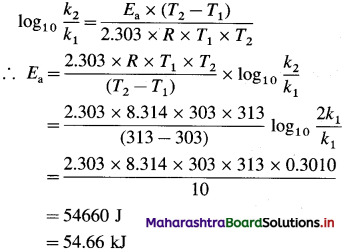
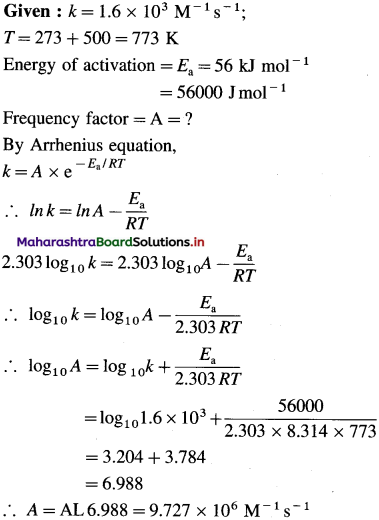
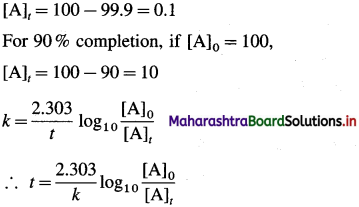
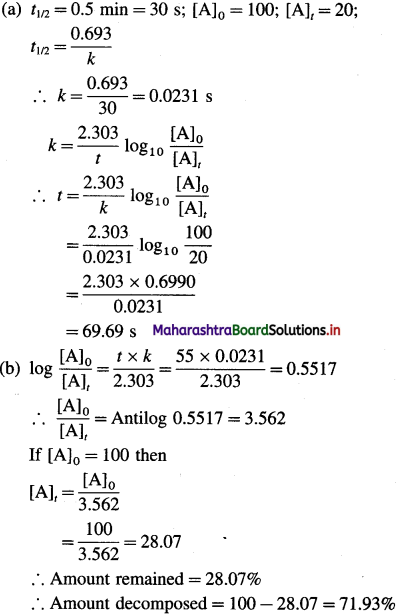
 where [A0] and [A]t are the respective initial and final concentrations of the reactant after time t.
where [A0] and [A]t are the respective initial and final concentrations of the reactant after time t.