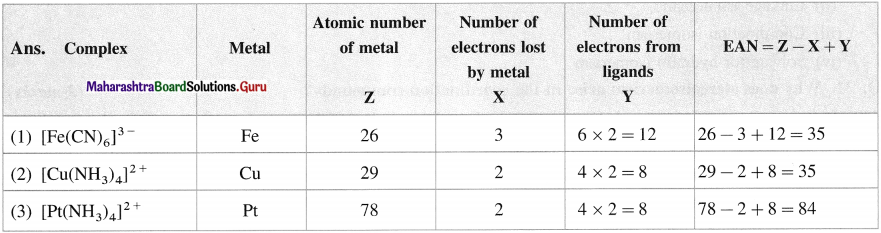
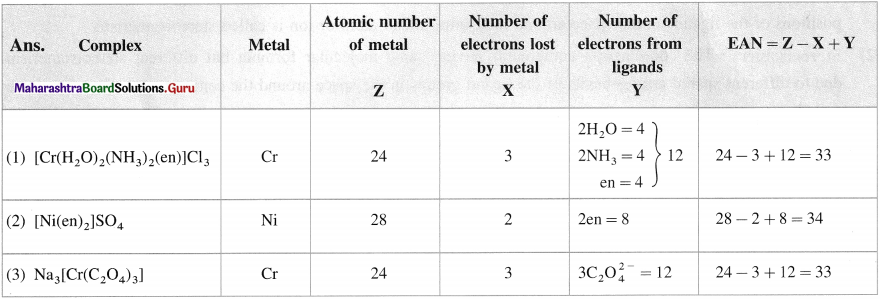
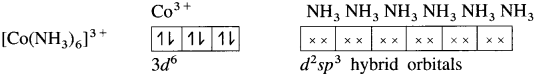
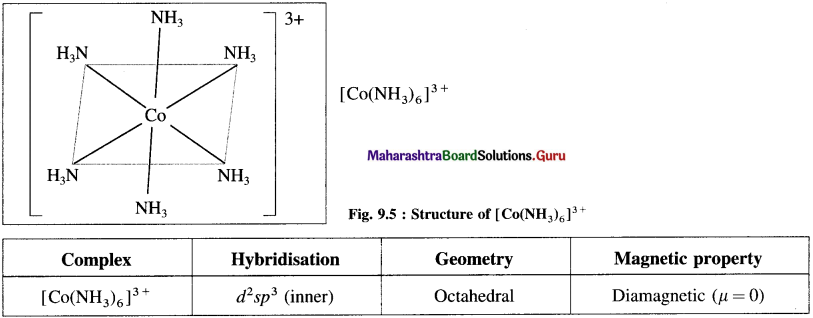
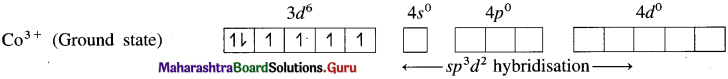
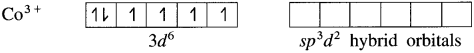
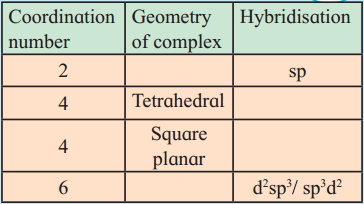
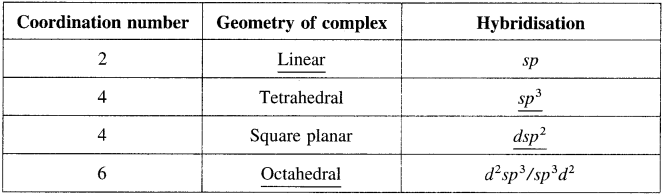
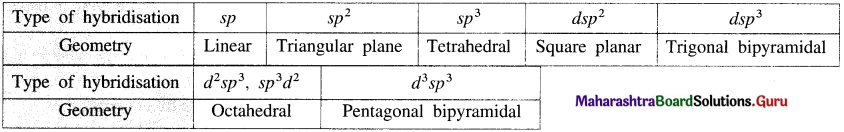
Balbharti Maharashtra State Board 12th Chemistry Important Questions Chapter 10 Halogen Derivatives Important Questions and Answers.
Maharashtra State Board 12th Chemistry Important Questions Chapter 10 Halogen Derivatives
Question 1.
What are halogen derivatives of hydrocarbons?
Answer:
The replacement of hydrogen atom/s in aliphatic or aromatic hydrocarbons by halogen atom/s results in the formation of halogen derivatives of hydrocarbons.
![]()
Question 2.
How are halogen derivatives of hydrocarbons classified?
Answer:
Halogen derivatives of alkane are classified as :
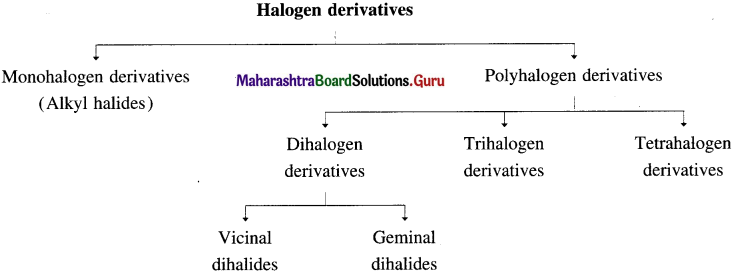
- Monohalogen derivative (or alkyl halide) : It is a halogen derivative of an alkane in which one hydrogen atom is replaced by one halogen atom and it is also called alkyl halide. E.g. C2H5Br.
- Poh halogen derivatives : These are halogen derivatives in which more than one hydrogen atoms of an alkane are substituted by corresponding number of halogen atoms.
They are classified as follows :
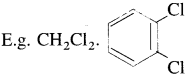
(i) Dihalogen derivatives : The compounds formed by the substitution of two hydrogen atoms of an alkane by two halogen atoms are called dihalogen derivatives.
They are further classified as :
- Vicinal dihalides
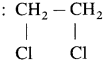 (Two halogen atoms on vicinal or adjacent carbon atoms)
(Two halogen atoms on vicinal or adjacent carbon atoms) - Geminal dihalides : CH3 – CHCI2 (Two halogen atoms on the same carbon atom)
(ii) Trihalogen derivatives : The compounds formed by the substitution of three hydrogen atoms of an alkane by three halogen atoms are called trihalogen derivatives.

(iii) Tetrahalogen derivatives : The compounds formed by the substitution of four hydrogen atoms of an alkane by four halogen atoms are called tetrahalogen derivatives. E.g. CCI4.
Question 3.
What are alkyl halides? How are they classified?
Answer:
The compound formed by the replacement of one hydrogen atom in an alkane by a halogen atom is called an alkyl halide. The halogen atom is bonded to sp3 hybridised carbon. Alkyl halides are classified into the following three classes depending on the type of the carbon to which halogen atom is bonded.
(1) Primary (1°) alkyl halide : Alkyl halide in which a halogen atom is bonded to a primary carbon atom is called primary alkyl halide.
[Primary (1°) carbon atom i.e., the carbon atom which is attached to only one carbon atom.]
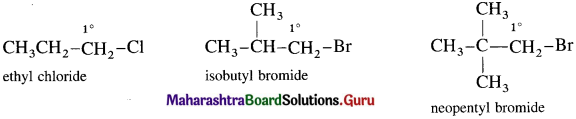
They are represented by the general formula R – 1°CH2 – X.
![]()
(2) Secondary (2°) alkyl halide : Alkyl halide in which a halogen atom is bonded to a secondary carbon atom is called secondary alkyl halide. [Secondary (2°) carbon i.e., the carbon atom which is attached to two other carbon atoms.]

They are represented by the general formula  (R and R’ can be same or different)
(R and R’ can be same or different)
(3) Tertiary (3°) alkyl halide : Alkyl halide in which halogen atom is bonded to a tertiary carbon atom is called tertiary alkyl halide. [Tertiary (3°) carbon i.e., the carbon atom which is attached to three other carbon atoms.]

They are represented by the general formula 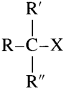 (R, R’ and R” may be same or different)
(R, R’ and R” may be same or different)
Question 4.
Explain the following :
(1) Alkyl halide or haloalkanes
(2) Allylic halides
(3) Benzylic halide
(4) Vinylic halide
(5) Haloalkyne
(6) Aryl halide or haloarenes.
Answer:
(1) Alkyl halide or haloalkanes : In alkyl halides or haloalkanes the halogen atom is bonded to sp3 hybridized carbon which is a part of saturated carbon chain.
Example : ![]()
(2) Allylic halides : In allylic halides, halogen atom is bonded to a sp3 hybridized carbon atom next to a carbon-carbon double bond.
Example : 
(3) Benzylic halide : In benzylic halides, halogen atom is bonded to a sp3 hybridized carbon atom which is further bonded to an aromatic ring.
Example : 
(4) Vinylic halides : In vinylic halides, halogen atom is bonded to a sp2 hybridized carbon atom of aliphatic chain. Vinylic halide is a haloalkene.
Example : 
(5) Haloalkyne : In haloalkynes, halogen atom is bonded to a sp hybridized carbon atom.
Example : ![]()
(6) Aryl halides or haloarenes : In aryl halides, halogen atom is directly bonded to the sp2 hybridized carbon atom of aromatic ring.
Example : 
![]()
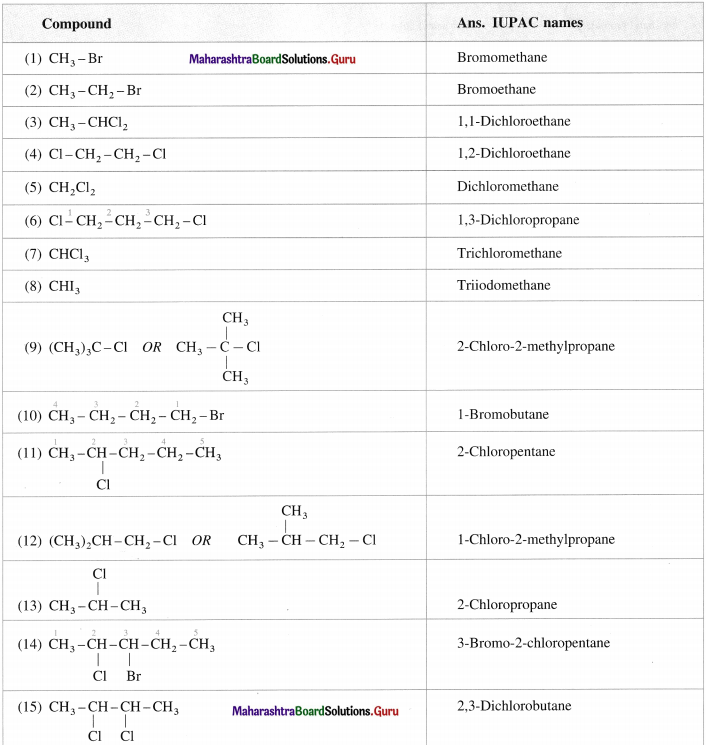
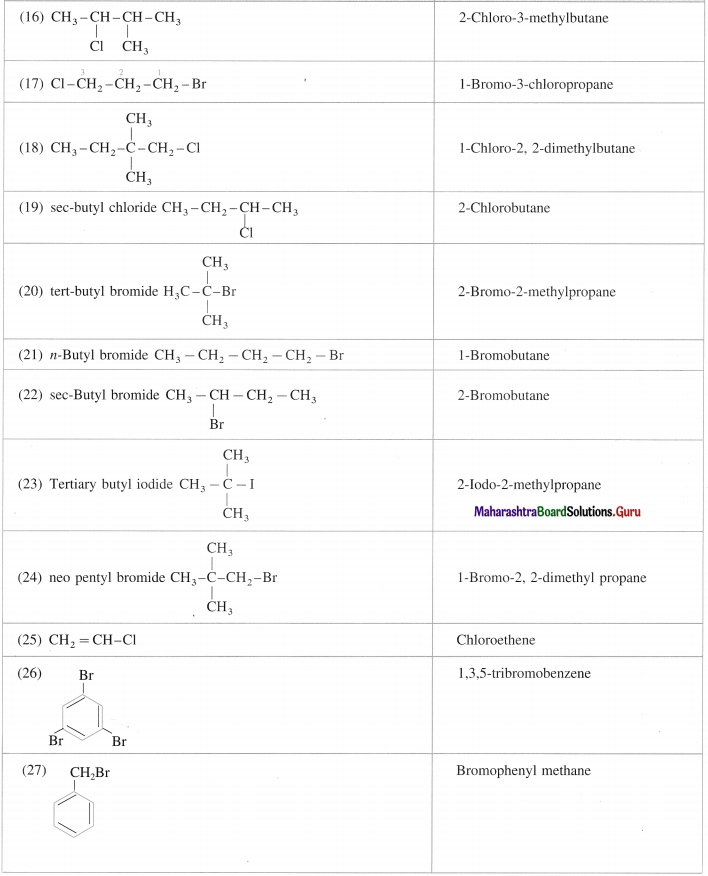
Question 5.
Give the IUPAC names of the following :
Answer:
![]()
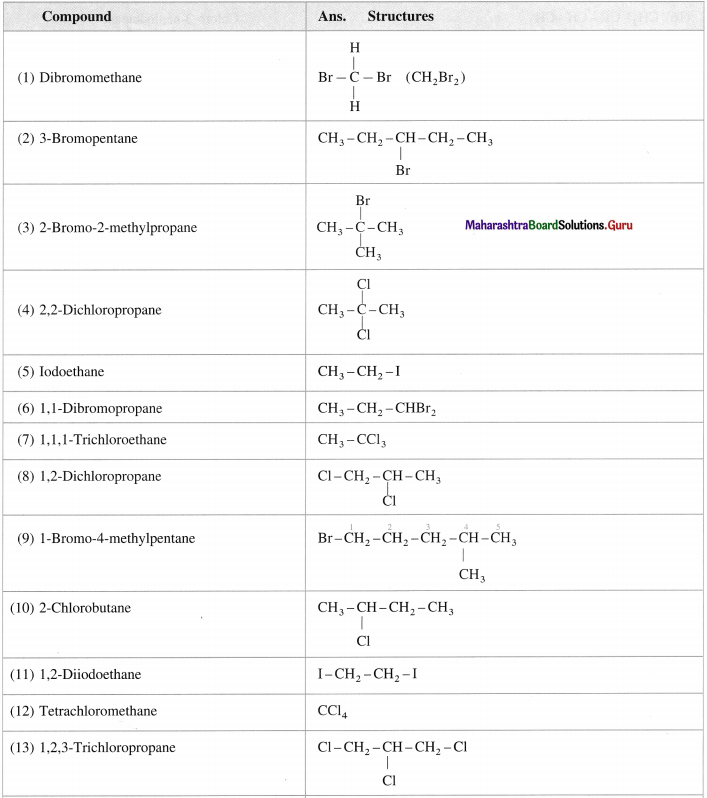
Question 6.
Draw the structures of the following compounds:
Answer:

![]()
Question 7.
Write the structure of-
(a) 3-chloro-3-ethylhex-l-ene
(b) 1-Iodo-2, 3-dimethylbutane
(c) 1, 3, 5-tribromobenzene
Answer:

Question 8.
Write structures of
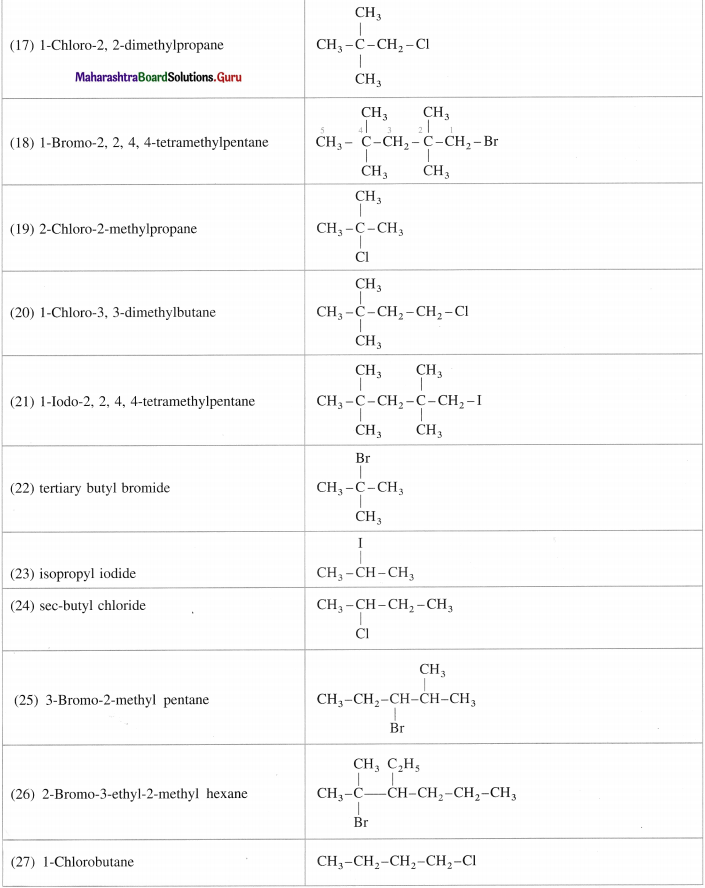
(a) 2-iodo-3-methyl pentane
(b) 3-chiorolleNane
(c) 1-chloro-2, 2-dimethyl propane
(d) 1-chloro-4-ethyl cyclohexane.
Answer:
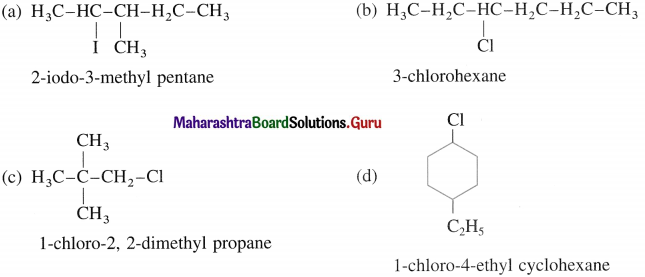
Question 9.
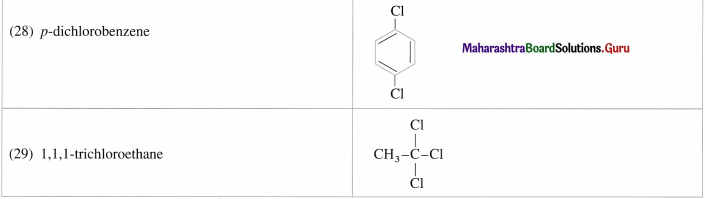
Write the possible isomers of monochloro derivatives of 2,3-Dimethylbutane and write their IUPAC names.
Answer:
The given parent hydrocarbon has molecular formula, C6H14. The monochloro derivative of this compound has molecular formula C6H13CI.
The parent hydrocarbon is,
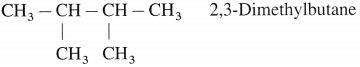
Hence the structures of isomers of monochioroderivative are.

![]()
Question 10.
Write structures and IUPAC names of all possible isomers of C5H11Br and classify them as l°/2°/3°.
Answer:
C5H11Br is a monohalogen derivative.

Question 11.
How are following compounds obtained from alcohols :
(1) ethyl chloride C2H5CI
(2) isopropyl chloride (CH3 CHCI – CH3)
(3) tert-butyl chloride (CH3)3 – CI?
Answer:
Alcohols in the presence of Lucas reagent which is a solution of concentrated HCI and ZnCI2 form alkyl halides. Hydrogen chloride is used with zinc chloride (Grooves’ process) for primary and secondary alcohols.

(3) Tertiary alcohols don’t need ZnCI2 to react with HCI.

The order of reactivity of alcohols with a given halo acid is 3° >2°> 1°.
Question 12.
How are following compounds prepared from alcohols:
(1) ethyl bromide (C2H5Br)
(2) isopropyl bromide (CH3 – CHBr – CH3)
(3) tert-butyl bromide (CH3)3 C – Br?
Answer:
(1) Ethyl alcohol on heating with conc. hydrobromic acid (48%) forms ethyl bromide.
OR
When ethyl alcohol is treated with a mixture of NaBr and H2SO4, ethyl bromide is formed. Here HBr is generated in situ.

(2) Isopropyl alcohol, on reaction with NaBr and dil. H2SO4 forms isopropyl bromide.
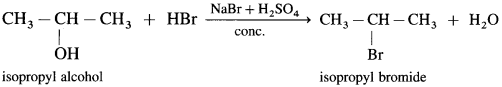
(3) Tertiary alcohol on reaction with sodium bromide and dil. H2SO4 forms tert-butyl bromide.

![]()
Question 13.
How is ethyl iodide obtained from ethyl alcohol?
Answer:
When ethyl alcohol is treated with sodium or potassium iodide in 95 % phosphoric acid, ethyl iodide is formed. Here HI is generated in situ.

Question 14.
How will you prepare the following :
(1) Ethyl chloride (chloroethane) from ethyl alcohol using
(i) PCI3
(ii) PCI5 and
(iii) SOCI2.
Answer:
(i) When ethyl alcohol is refluxed with phosphorus trichloride, ethyl chloride is formed.

(ii) When ethyl alcohol is refluxed with phosphorus pentachloride, ethyl chloride is formed.
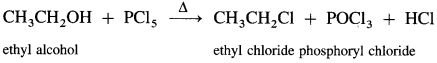
(iii) When ethyl alcohol is refluxed with thionyl chloride, in the presence of pyridine, ethyl chloride is formed. The by-products obtained are gases. Therefore, this method is preferred for preparation of alkyl chloride.
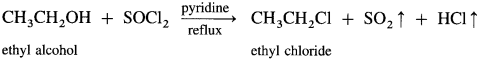
(2) Isopropyl chloride (2-chloropropane) from isopropyl alcohol using
(i) PCI3
(ii) PCI5
(iii) SOCI2.
Answer:
When isopropyl alcohol is refluxed with phosphorus trichloride, isopropyl chloride is formed.

When isopropyl alcohol is refluxed with phosphorus pentachloride, isopropyl chloride is formed.

When isopropyl alcohol is refluxed with thionyl chloride, in the presence of pyridine, isopropyl chloride is formed. The by-products obtained are gases. Therefore, this method is preferred for the preparation of alkyl chloride.
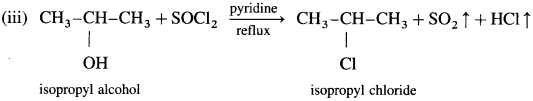
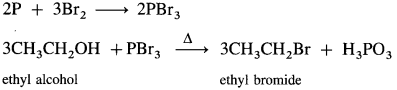
(3) Ethyl bromide (bromoethane) from ethyl alcohol.
Answer:
When ethyl alcohol is treated with a mixture of red phosphorus and bromine or hydrobromic acid (phosphorus tribromide is generated in situ), ethyl bromide is formed.
![]()
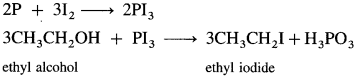
(4) Ethyl iodide (do ethane) from ethyl alcohol.
Answer:
When ethyl alcohol is heated with a mixture of red phosphorus and iodine, (phosphorus triiodide is generated in situ), ethyl iodide is formed.
Question 15.
Explain halogenation of methane.
Answer:
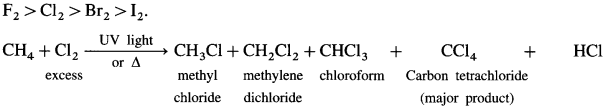
Halogenation : A reaction of alkanes with halogens (CI2, Br2, I2) in the presence of appropriate conditions forming a mixture of alkyl halides.
(1) Chlorination:

(2) When excess of chlorine is used, tetrachioro methane, a major product is obtained. When excess of methane is used, chioromethane, a major product is obtained. The order of reactivity of halogens towards alkane is
(3) lodination:

However, iodination reaction is a reversible reaction. HI being a strongest reducing agent reduces methyl iodide back to methane.
(4) Fluorination: A reaction of alkane with fluorine is explosive and also hydrofluoric acid is poisonous and corrosive. Hence, alkyl fluorides are not prepared by halogenation of alkane.
Question 16.
Predict the possible products of the following reaction :
(1) Bromination of propane
(2) Bromination of n-butane
(3) Bromination of 2-Methyl propane.
Answer:
(1) Bromination of propane
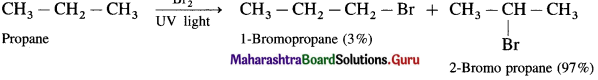
(2) Bromination of n-butane
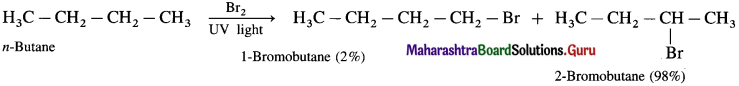
(3) Bromination of 2-Methyl propane
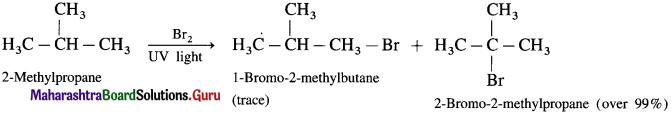
![]()
Question 17.
How are following compounds prepared by halogenation of ethane :
(1) Chloroethane
(2) Bromoethane
(3) Iodoethane?
Answer:
(1) Chlorination of ethane : When ethane (excess) is reacted with a limited quantity of chlorine in the presence of diffused sunlight or U.V. light or at high temperature, chloroethane is obtained.
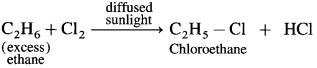
(2) Bromination of ethane : When ethane is heated with Br2 in the presence of anhydrous AlBr3, bromoethane is obtained.
![]()
(3) Iodination : When ethane is reacted with I2 in the presence of suitable oxidising agents like-HgO or HIO3 or dilute HNO3 iodoethane is obtained.
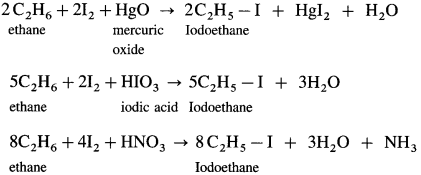
Question 18.
Direct iodination of alkanes is not possible.
Answer:
(1) Direct iodination of alkanes using iodine is highly reversible.
\(\mathrm{RH}+\mathrm{I}_{2} \rightleftharpoons \mathrm{RI}+\mathrm{HI}\)
(2) Hydroiodic acid HI being strong reducing agent, it reduces RI to alkane RH.
(3) The reaction takes place only in the presence of a suitable oxidizing agent like HgO, HIO3 or dilute HNO3 which decomposes HI. Hence, direct iodination of alkanes is not possible.
![]()
Question 20.
How are following compounds obtained from alkenes :

Answer:
(1) Ethene on reaction with hydrogen chloride forms
![]()
(2) Ethene on reaction with hydrogen bromide forms
![]()
(3) Propene on reaction with hydrogen iodide forms

(4) but-2-ene on reaction with hydrogen iodide forms 2-iodobutane.

Question 19.
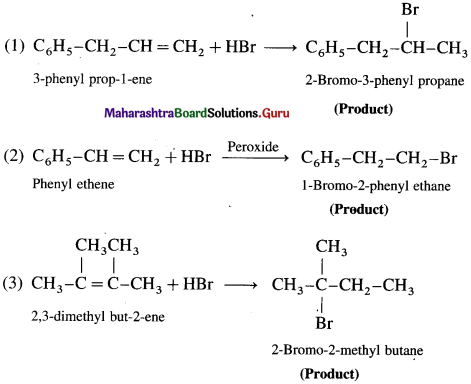
State and explain Markovnikov’s rule.
Answer:
Markovnikov’s rule : When an unsymmetrical reagent is added to an unsymmetrical alkene, the negative part of the reagent gets attached to that carbon atom of the double bond which carries less number of hydrogen atoms.
Example : Addition of HBr’to unsymmetrical alkene like propene gives two products.

![]()
Isopropyl bromide is the major product, since the negative part (Br–) of HBr is attached to carbon atom of a double bond with less number of hydrogen atoms.
Question 20.
Explain peroxide effect.
OR
Write a note on the Kharasch-Mayo effect.
OR
Explain the addition of HBr to (unsymmetrical alkene) propane in the presence of benzoyl peroxide.
Answer:
The addition of HBr to an unsymmetrical alkene (propane) in the presence of benzoyl peroxide takes place in the opposite orientation to that of Markovnikov’s rule and this is known as Kharasch-Mayo effect or peroxide effect or Anti-Markovnikov addition.

Question 21.
Write the structure of alkyl halide obtained by the action hydrogen bromide on 2-Methyiprop-1-ene in the presence of peroxide.
Answer:
In the presence of peroxide. HBr to 2-Methyl prop-I-cne forms l-Bromo-2-methylpropane.
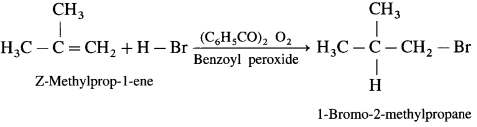
Question 22.
How are alkyl iodides prepared from alkyl chlorides/bromides?
Answer:
Alkyl iodide is prepared by treating alkyl chloride or alkyl bromide with sodium iodide, in the presence of dry acetone, sodium chloride or sodium bromide precipitates from the solution and can be separated by filtration. This reaction is known as Finkelstein reaction.
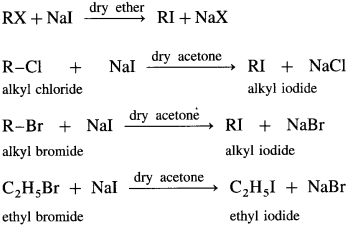
Question 23.
How are alkyl fluorides prepared with alkyl chlorides/alkyl bromides?
Answer:
When alkyl chloride or alkyl bromide is heated with metallic fluorides like AgF, CaF2, CoF2 or Hg2F2, alkyl fluoride is formed.
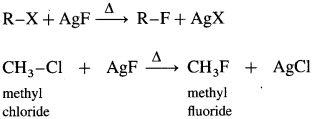
This reaction is known as Swarts reaction.
Question 24.
Explain the preparation of haloarenes using electrophilic substitution.
Answer:
When arene is treated with chlorine or bromine in dark at ordinary temperature in the presence of lewis acid as a catalyst like Fe, FeCI3 or anhydrous AlCI3, aryl chloride or aryl bromide is formed.
When toluene is brominated in dark at ordinary temperature in the presence of iron, a mixture of ortho and para bromo tolerene is obtained.

Ortho and para isomers can be easily separated as there is large difference in melting points of ortho and para isomers.
![]()
Question 25.
Write a note on Sandmeyer’s reaction.
Answer:
Aryl halides are most commonly prepared by replacement of nitrogen of diazonium salt. The replacement of diazonium group by -Cl or -Br using cuprous salt is called Sandmeyer’s reaction. When a primary aromatic amine (like aniline) suspended in cold F1C1, is treated with sodium nitrite, a diazonium salt (benzene diazonium chloride) is formed. When diazonium salt is treated with cuprous chloride or cuprous bromide, aryl halide (chlorobenzene or bromobenzene) is formed.

When benzene diazonium salt is mixed with potassium iodide, iodobenzene is formed.
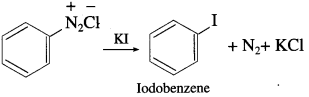
Question 26.
Define the following :
Answer:
(1) Monochromatic light : It consists of rays of single wavelength vibrating in different planes perpendicular to the direction of propagation of the light.
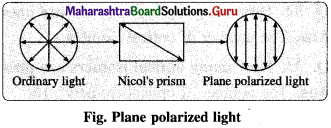
(2) Plane polarized light : A light having oscillations only in one plane perpendicular to direction of propagation of light is known as plane polarized light.
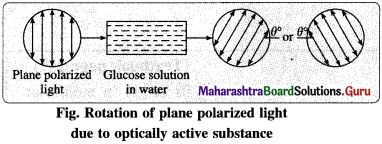
(3) Optical isomerism : The steroisomerism in which the isomers have different spatial arrangements of groups/atoms around a chiral atom is called optical isomerism.
(4) Optical activity : The property of a substance by which it rotates plane of polarization of incident plane polarized light is known as optical activity.
(5) Optically active compound : The compound which rotate the plane of plane polarized light is called optically active compound.
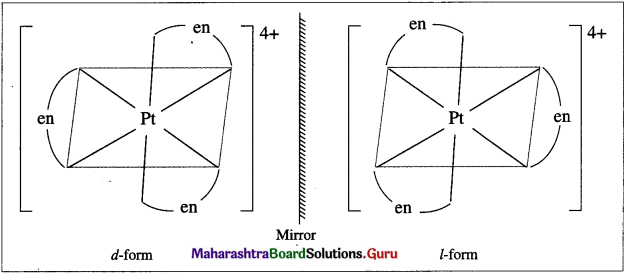
(6) Enantiomers : The optical isomers which are non-superimposable mirror images of each other are called enantiomers or enantiomorphs or optical antipodes.
Example : 2-chlorobutane, lactic acid
(7) Chiral carbon atom : Carbon atom in a molecule which carries four different groups/atoms is called chiral carbon atom.
Chiral atom in a molecule is marked with asterisk (*)
For example : C in lactic acid
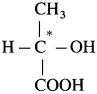
(8) Chiral molecule : When a molecule contains one chiral atom, it acquires a unique property i.e. it is non- superimposable with its mirror image is said to be chiral molecule.
(9) Chirality : The relationship between a chiral molecule and its mirror image is similar to the relationship between left and right hands. Therefore it is called handedness or chirality.
(10) Dextrorotatory substance or r/-Isomer : An optically active substance (or isomer) which rotates the plane of a plane polarized light to the right hand side (RHS) is called dextrorotatory substance (or isomer) and denoted by d or (+) sign.
(11) Laevorotatory substance or /-Isomer : An optically active substance (or isomer) which rotates the plane of a plane polarized light to the left hand side (LHS) is called laevorotatory substance (or isomer) and denoted by / or (-) sign.
(12) Racemic mixture or Racemate : A mixture containing equimolar quantities of dextro (d) and laevo (/) optical
isomers which is optically inactive due to molecules of one enantiomer is cancelled by equal and opposite optical rotation due to molecules of the other enantiomer is called a racemic mixture or racemate. It is represented as (dl) or (+).
![]()
Question 27.
Calculate the number of isomers for 2-chlorobutane.
Answer:
The number of optical isomers possible for a compound is 2n where n = number of asymetric carbon atoms.
As n = 1 for 2-ehlorobutane, 2n = 21 = 2. Hence, it has two optical isomers.
Question 28.
How many optical isomers are possible for C5H11 CI?
Answer:
The number of optical isomers : 3.
Question 29.
How many optical isomers are possible for glucose?
Answer:
The number of optical isomers : 16.
Question 30.
Draw the structures and indicate the chiral carbon atoms in
(1) Lactic acid
(2) 2-Chlorobutane.
Answer:
(1) In lactic acid structure, 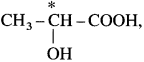 the starred carbon atom is chiral carbon atom as it is attached to four different substituents, COOH, OH, CH3 and H.
the starred carbon atom is chiral carbon atom as it is attached to four different substituents, COOH, OH, CH3 and H.
(2) In 2-chlorobutane structure, 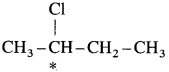 the starred carbon atom is chiral carbon atom as it is attached to four different substituents, -CH2 – CH3 (ethyl), CH3 (methyl), Cl and H.
the starred carbon atom is chiral carbon atom as it is attached to four different substituents, -CH2 – CH3 (ethyl), CH3 (methyl), Cl and H.
Question 31.
Identify chira! and achiral molecules.

Question 32.
Complete the following reactions and explain optical activity of the products formed:
(i) Pent-1-ene with HBr
Answer:
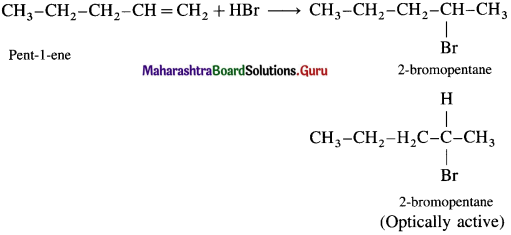
(ii) Pent-2-ene with HBr
Answer:

![]()
Question 33.
C6H12 (A) on treatment with HCI produced a compound Y. Which is optically active, what is structure A?
Answer:

Question 34.
A racemic mixture is optically inactive. Explain.
Answer:
- A racemic mixture contains equimolar (or equimolecular) quantities of the dextrorotatory (d-) and laevorotatory (l-) isomers (enantiomers) of a compound.
- The d-enantiomer rotates the plane of plane-polarized light to the right, while the l-enantiomer rotates the same to the left to the same extent.
- The quantities of the d- and l-enantiomers being the same, both the rotations are of the same magnitude, but of opposite directions. Hence, they cancel each other. Hence, a racemic mixture is optically inactive.
- It is represented as dl or ( + ). Example : ( ± ) lactic acid
Question 35.
Explain Fischer projection formula with illustration.
OR
Write a note on Fischer projection formula.
Answer:
Fischer projection formula or cross formula : The three dimensional (3-D) view of a molecule is presented on plane of paper. A Fischer projection formula can be drawn by visualizing the main carbon chain verical in the molecule. Each carbon on the vertical chain is represented by a cross.

Conventionally the horizontal lines of the cross represent bonds projecting up from the carbon and the vertical lines represent the bonds going below the carbon.
Question 36.
Explain Wedge formula with illustration
OR
Write a note on Wedge formula.
Answer:
Wedge formula : When a tetrahedral carbon is imagined to be present in the plane of paper all the four bonds at this carbon cannot lie in the same plane. The bonds in the plane of paper are represented by normal lines, the bonds projecting above the plane of paper are represented by solid wedges (or simply by bold lines) while bonds going below the plane of paper are represented by broken wedges (or simply by broken lines).

Question 37.
Give a laboratory test to confirm the presence of halogen in the original organic compound.
Answer:
Haloalkanes are of neutral type in aqueous medium. On warming with aqueous sodium or potassium hydroxide the covalently bonded halogen in haloalkane is converted to halide ion.
![]()
When this reaction mixture is acidified by adding dilute nitric acid and silver nitrate solution is added a precipitate of silver halide is formed which confirms presence of halogen in the original organic compound.
![]()
![]()
Question 38.
Define the following :
Answer:
(1) Mechanism of a reaction : It is a step by step description of exactly how the reactants are transformed into products in as much details as possible.
(2) Substitution reaction : When a group bonded to a carbon in a substrate is replaced by another group to get a product with no change in state of hybridization of that carbon, the reaction is called substitution reaction.
Question 39.
Describe the action of aqueous KOH (or NaOH) on :
(1) ethyl bromide
(2) isopropyl bromide
(3) tert-butyl chloride
(4) methyl bromide
(5) 2-chlorobutane.
Answer:
(1) Ethyl bromide : When ethyl bromide (bromoethane) is refluxed with aqueous potassium hydroxide, ethyl alcohol is formed. The reaction is called a hydrolysis reaction.

(2) Isopropyl bromide : When isopropyl bromide (2-bromopropane) is boiled with aqueous potassium hydroxide, isopropyl alcohol is formed.

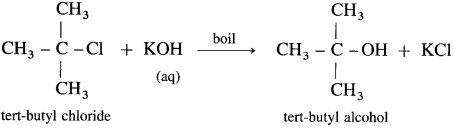
(3) Tert-butyl chloride : When tert-butyl chloride is refluxed with aqueous potassium hydroxide, tert-butyl alcohol is formed.
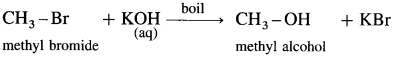
(4) Methyl bromide : When methyl bromide (bromomethane) is heated with aq. KOH, it is hydrolysed to methyl alcohol (methanol).
(5) 2-chlorobutane : When 2-Chlorobutane is boiled with aqueous KOH, Butan-2-ol is obtained.
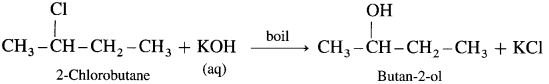
Question 40.
Describe the action of sodium ethoxide on
(1) ethyl bromide
(2) methyl bromide :
OR
Write a note on Williamson’s synthesis.
OR
How are ethers prepared from alkyl halides?
Answer:
Williamson’s synthesis : When an alkyl halide (R – X) is heated with sodium alkoxide (R – O – Na), an ether is obtained. In this reaction halide (-X) of alkyl halide is replaced by an alkoxy group (-OR). This reaction is known as Williamson’s synthesis. This method is used to prepare simple (or symmetrical) ethers and mixed (or unsymmetrical) ethers.
Sodium alkoxide is obtained by a reaction of sodium with an alcohol.
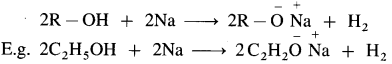
(1) Simple (symmetrical) ether : When an alkyl halide and sodium alkoxide having similar alkyl groups are heated, symmetrical ether is obtained.
![]()
e.g., When ethyl bromide is heated with sodium ethoxide, diethyl ether is formed.
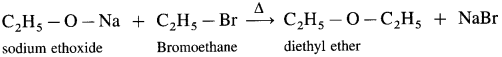
(2) Mixed (unsymmetrical) ether : When an alkyl halide and sodium alkoxide having different alkyl groups are heated, unsymmetrical ether is obtained.
![]()
When methyl bromide is heated sodium ethoxide, ethyl methyl ether is formed.
When ethyl bromide is heated with sodium meihoxide, ethyl methyl ether is formed.

![]()
Question 41.
What is the action of silver salt of carboxylic acid on alkyl halide?
Answer:
When an alkyl halide (R – X) is heated with silver salt of carboxylic acid (R -COOAg). an ester is obtained.

Question 42.
Describe the action of alcoholic silver acetate on
(1) methyl bromide
(2) ethyl bromide.
Answer:
(1) Methyl bromide : When methyl bromide is heated with an alcoholic silver acetate, methyl acetate is formed.
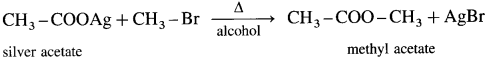
(2) Ethyl bromide : When ethyl bromide is heated with an alcoholic silver acetate, ethyl acetate is formed.
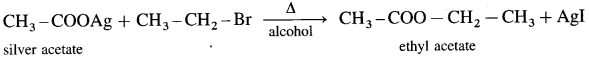
Question 43.
What is the action of alcoholic silver propionate on ethyl bromide?
Answer:
When ethyl bromide is heated with an alcoholic silver propionate. ethyl propionate is formed.
![]()
Question 44.
Describe the action of excess of ammonia on (I) ethyl bromide (2) n.propyl bromide.
Answer:
(1) Ethyl bromide : When ethyl bromide is boiled under pressure with an excess of alcoholic ammonia, ethylamine (ethanamine) is formed. This is known as ‘ammonolysis of ethyl bromide.

(2) n-propyl bromide : When n-propy1 bromide is boiled under pressure with an excess of ammonia, n-propyl amine (propanamine) is formed.

Question 45.
What is ammonolysis? Give a suitable example for the reaction.
Answer:
When an alkyl halide is boiled under pressure with an excess of alcoholic solution of ammonia (NH3), corresponding (primary amine) alkyl amine is formed. This reaction is known as ammonolysis of alkyl halide.

(1) Ethyl bromide : When ethyl bromide (bromoethane) is refluxed with aqueous potassium hydroxide, ethyl alcohol is formed. The reaction is called a hydrolysis reaction.

(2) Isopropyl bromide : When isopropyl bromide (2-bromopropane) is boiled with aqueous potassium hydroxide, isopropyl alcohol is formed.

(3) Tert-butyl chloride : When tert-butyl chloride is refluxed with aqueous potassium hydroxide, tert-butyl alcohol is formed.

(4) Methyl bromide : When methyl bromide (bromomethane) is heated with aq. KOH, it is hydrolysed to methyl alcohol (methanol).

(5) 2-chlorobutane : When 2-Chlorobutane is boiled with aqueous KOH, Butan-2-ol is obtained.

![]()
Question 46.
Describe the action of aqueous alcoholic potassium cyanide on
(1) ethyl bromide
(2) methyl iodide.
Answer:
Ethyl bromide : When ethyl bromide (bromoethane) is boiled with alcoholic solution of potassium cyanide in aqueous ethanol, ethyl cyanide (ethyl nitrile) is formed.
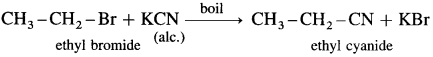
(2) Methyl iodide : When methyl iodide is boiled with alcoholic solution of potassium cyanide, methyl cyanide is formed.
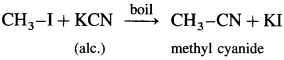
Question 47.
Describe the action of alcoholic silver cyanide on
(1) ethyl bromide
(2) methyl chloride.
OR
Explain isocyanide reaction of
(1) ethyl bromide
(2) methyl chloride.
Answer:
(1) Ethyl bromide : When ethyl bromide is heated with alcoholic silver cyanide, ethyl isocyanide is formed.
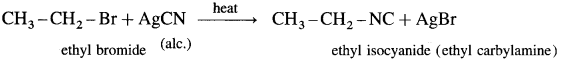
(2) Methyl chloride : When .methyl chloride is heated with alcoholic silver cyanide, methyl isocyanide is formed.
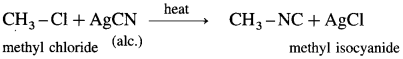
The above reactions (1) and (2) are called isocyanide reaction.
Question 48.
Describe the action of potassium nitrite on
(i) ethyl bromide,
(ii) methyl chloride.
Answer:
(1) Ethyl bromide : When ethyl bromide is treated with potassium nitrite, ethyl nitrite is formed.

(2) Methyl chloride : When methyl chloride is treated with potassium nitrite, methyl nitrite is formed.

Question 49.
Describe the action of silver nitrite on (1) ethyl chloride (2) n-propyl bromide.
Answer:
(1) Ethy chloride : When ethyl chloride is treated with silver nitrite, nitroethane is obtained.
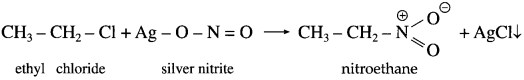
(2) n-Propyt bromide: When n-propyl bromide is treated with silver nitrate, nitropropane is obtained.

Question 50.
How will you convert (the following:
(1) Ethyl bromide to ethanol.
Answer:
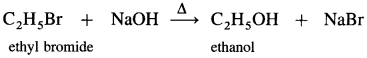
(2) Ethyl bromide to propane nitrile.
Answer:
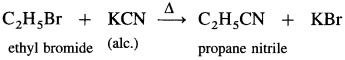
(3) Ethyl bromide to ethyl amine.
Answer:
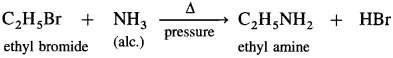
(4) Ethyl bromide to ethyl acetate.
Answer:
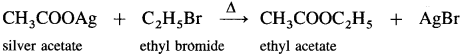
(5) Ethyl bromide to ethyl isocyanide.
Answer:

(6) Ethyl bromide to ethyl methyl ether.
Answer:
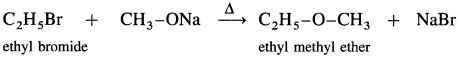
![]()
(7) Ethyl bromide to n-butane.
Answer:

(8) Ethyl bromide to Ethyl magnesium bromide.
Answer:
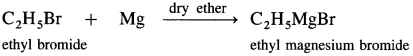
Question 51.
Define the following :
Answer:
(1) Nucleophilic bimolecular reaction (SN2) : The substitution reaction in which a nucleophile reacts with the substrate and the rate of the reaction depends on the concentration of the substrate and the nucleophile is called a nucleophilic bimolecular reaction.
Example :
![]()
(2) SN1 reaction : The substitution reaction in which a nucleophile reacts with the substrate and the rate of the reaction depends only on the concentration of the substrate is called nucleophilic unimolecular or first order reaction or SN1 reaction.
Question 52.
Explain, the mechanism of alkaline hydrolysis (reaction with aqueous KOH) of tert-butyl bromide (2-Bromo-2-methylpropane) with energy profile diagram.
OR
Explain only reaction mechanism for alkaline hydrolysis of tert-butyl bromide.
Answer:
(i) Consider alkaline hydrolysis of tert-butyl bromide (2-Bromo-2 methylpropane) with aqueous NaOH or KOH.
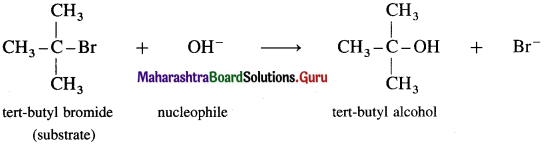
(ii) Kinetics of the reaction : Due to steric hindrance of voluminous three methyl groups around carbon, nucleophile OH- cannot attack carbon atom directly. Hence, the reaction takes place in two steps.
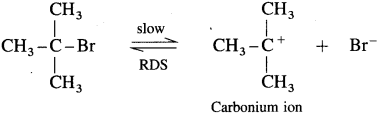
Step I : This involves heterolytic fission of C – Br covalent bond in the substrate forming carbocation and Br–. This is a slow process.
Step II : This step involves attack of nucleophile OH- or carbocation forming C – OH bond and product tert-butyl alcohol. Since it involves ionic charge neutralisation, it is a fast step.
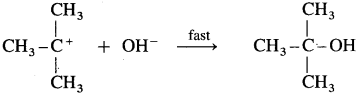
Rate Determining Step (R.D.S.) : Since the first step is a slow step, it is R.D.S., and therefore the rate of the reaction depends on the concentration of only one reactant, (CH3) C – Br.
Rate = R = k [(CH3)3 C – Br] where k is a rate constant of the reaction.
SN1 reaction : The reaction between tert.butyl bromide and hydroxide ion to form tert.butyl alcohol follows a first-order kinetics. The rate of this reaction depends only on the concentration of one substance (tert-butyl bromide) and is independent of the concentration of alkali added. It is an unimolecular first (1st) order Nucleophilic Substitution reaction denoted as SN reaction.
![]()
Stereochemistry and mechanism of the reaction : The reaction takes place in two steps and both the steps involve formation of transition states (T.S.).
T.S. -1 for first step :

In this transition state, C – Br bond is partially broken, so that carbon atom carries partial positive charge (+δ) and Br carries partial negative charge (-δ) which further breaks forming carbocation and Br . Tert-butyl cation (carbocation) has a planar structure and the CH3 – C – CH3 bond angle is 120°. It is the intermediate of the reaction. It is unstable. In this step, hybridisation of carbon atom changes from sp3 (tetrahedral geometry) to sp (planar geometry).
T.S. – II for second step :

In this transition state, C – OH bond is partially fonned so that carbon atom carries partial positive charge (+ δ) and OH carries partial negative charge ( -δ) which further forms tert-butyl alcohol.
Formation of a racemic mixture : Since OH– has equal probability of the attack on carbocation from frontside and from backside, the products obtained are equal. In case of optical active alkyl halide, a racemic mixture is obtained.
Question 53.
Discuss SN2 mechanism of methyl bromide using aqueous KOH. Draw energy profile diagram.
OR
Discuss the mechanism of alkaline hydrolysis of methyl bromide or Bromomethane.
Answer:
(1) Consider alkaline hydrolysis of methyl bromide (Bromomethane). CH3Br with aqueous NaOH or KOH.

(2) Stereochemistry and Kinetics of the reaction iR.D.S.) : This hydrolysis reaction takes place only in one step which is a rate determining step i.e. R.D.S. The rate of hydrolysis reaction depends on the Concentration of CH3Br
and 0H which are present in the R.D.S. of the reaction.
Rate = R = k [CH3Br] (OH]
where k is rate constant of the reaction.
SN2 reaction : The reaction between methyl bromide and hydroxide ion to form methanol follows a second order kinetics, since the rate of the reaction depends on the concentrations of two reacting species, namely methyl bromide and hydroxide ion it is bimolecular second order (2nd) Nucleophilic Substitution reaction denoted by SN2.
(3) Mechanism of the reaction :
(i) It is a single step mechanism. The reaction takes place in the following steps :
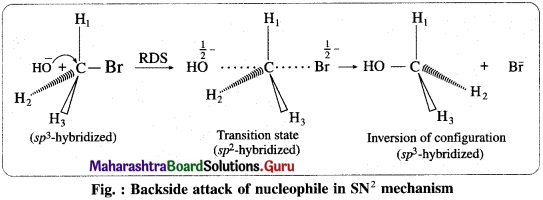
(ii) Backside attack of the nucleophile : Nucleophile, OH– attacks carbon atom of CH3Br from back side i. e. from opposite side to that of the leaving group i.e. Br– to experience minimum steric repulsion and electrostatic repulsion between the incoming nucleophile (OH–) and leaving Br–.
![]()
(iii) Transition state : When a nucleophile, OH– approaches carbon atom of CH3Br, the potential energy of the system increases until a transition state (T.S.) of maximum potential energy is formed in which C – Br bond is partially broken and C – OH bond is partially formed. The negative charge is equally shared by both incoming nucleophile- OH– and outgoing, leaving group-Br–. (Thus, the total negative charge is diffused.)
(iv) In CH3Br, carbon atom is sp3 -hybridized and CH3Br molecule is tetrahedral. The hybridisation of carbon atom changes to sp2 hybridisation. The transition state contains pentacoordinate carbon having three δ (sigma) bonds in one plane making bond angles of 120° with each other i.e., H1; H2 and H3 atoms lie in one plane while two partial covalent bonds containing Br and OH lie collinear and on opposite sides perpendicular to the plane.
(v) Inversion of configuration : The transition state decomposes fast by the complete breaking of the C-Br bond and the new C-OH bond is formed on the other side. The breaking of C-Br bond and the formation of C-OH bond take place simultaneously. The energy required to break the C-Br bond is partly obtained from the energy released when C-OH bond is formed. The formation of product CH3OH is accompanied by complete or 100% inversion of configuration forming again sp3-hybridized carbon atom giving tetrahedral CH3OH molecule. But in this structure the positions of H2 and H3 atoms in the reactant (CH3Br) and in product are on the opposite side. This inversion of configuration is called Walden inversion.
Question 54.
Discuss the factors influencing SN1 and SN2 mechanism.
Answer:
(1) Nature of substrate : SN2 : The transition state (T.S.) of SN2 mechanism + is pentacoordinate, it is crowded. As a result SN2 mechanism is favoured in primary halides and least favoured in tertiary halides.
SN1 : A planar carbocation intermediate is formed in SN1 reaction. Bulky alkyl groups can be easily accommodated in planar carbocation and it has no steric crowding. As a result SN1 mechanism is favoured in tertiary halides and least favoured in primary halides.
The carbocation intermediate is stabilized by + effect of alkyl substituents and also by hyperconjugation y effect of alkyl substituents containing a-hydrogens. As a result, SN1 mechanism is favoured in tertiary halides and least favoured in primary halides.

Thus, tertiary alkyl halides undergo nucleophilic substitution by SN1 mechanism while primary halides follow SN2 mechanism.
(2) Nucleophilicity of the reagent : A strong nucleophile attacks the substrate faster and favours SN2 mechanism. The rate of SN1 mechanism is independent of the nature of nucleophile. Nucleophile does not react in the 1st step (slow step) of SN1. Nucleophile reacts fast after the carbocation intermediate is formed.
(3) Solvent polarity : (1) SN1 reaction proceeds more rapidly in polar protic solvents than in aprotic solvent. Polar protic solvent decreases the rate of SN2 reaction. (2) In SN2 mechanism, rate depends on substrate as well as nucleophile. A polar solvent stabilizes nucleophile by solvation. Thus solvent deactivates the nucleophile by stabilizing it. Hence, aprotic solvents or solvent of low polarity will favour SN2 mechanism.
Question 55.
How does relative reactivity for alkaline hydrolysis with respect to SN2 and SN1 vary in the following alkyl halides :
(1) Bromomethane
(2) Bromoethane
(3) 2-Bromopropane
(4) 2-Bromo-2-methylpropane ?
Answer:
(A) Relative reactivity for SN2 mechanism decreases in the order of :
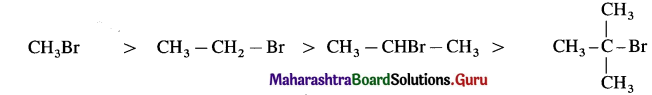
(B) Relative reactivity for SN1 mechanism decreases in the order of :
2-Bromo-2-methylpropane > 2-Bromopropane > Bromoethane > Bromomethane
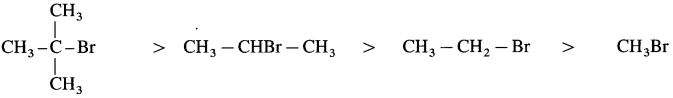
![]()
Question 56.
Explain with reason the relative order of reactivity of l°/2°/3° alkyl halides by SN1 mechanism.
Answer:
In alkaline hydrolysis of an alkyl halide by SN1 mechanism, the formation of carbocation as an intermediate product is involved.
The increasing order of a stability of carbocation is,

The stability order for carbocation is 3° > 2° > 1°.
Therefore the increasing order of reactivity by SN1 mechanism of alkyl halides is
(1°) primary < (2°) secondary < (3°) tertiary
Question 57.
Which one of the following is more easily hydrolysed in SN1 and SN2 reaction by aqueous KOH, C6H5 CHCIC6H5 and C6H5CH2CI?
Answer:
In SN1 reaction C6H5CHCI C6H5 will be more easily hydrolysed than C6H5CH2CI
In SN2 reaction C6H5 CH2CI will be more easily hydrolysed than C6H5CHCIC6H5.
Question 58.
Choose the member that will react faster than the following pairs by SN1 mechanism.
(1) l-bromo-2, 2-dimethyl propane or 2-bromopropane.

Answer:
The reactivity of SN1 reaction depends on the steric hindrance, in 2-bromopropane, a-carbon atom is attached to two methyl groups suffers greater steric hindrance to nucleophilic attack than l-bromo-2, 2-dimethyl propane. Hence, 2-bromopropane react faster by SN1 mechanism.
(2) 2-Iodo-2-methyl butane or 2-iodio-3-methyl butane.

Answer:
Since, 2-Iodo-2-methyl butane is a tertiary alkyl halide, it undergoes SN-1 reaction faster than 2-iodo-3-methyl butane.
(3) 1-Chloro propane or 2-chloropropane.

Answer:
Since, 2-chloropropane is a secondary alkyl halide, it undergoes SN-1 reaction faster than 1-chloropropane.
(4) 2-Iodo-2-methyl butane or tert-butyl chloride.

Answer:
Since, iodine is a better leaving group than chloride 2-iodo-2-methyl butane undergo SN-1 reaction faster than tert-butyl chloride.
![]()
Question 59.
Write a note on elimination reaction.
OR
Explain dehydrohalogenation reaction.
Answer:
When alkyl halide having at least one β-hydrogen is boiled with alcoholic solution of potassium hydroxide (KOH), an alkene is formed due to elimination of hydrogen atom from β-carbon and halogen atom from α-carbon, is called dehydrohalogenation.
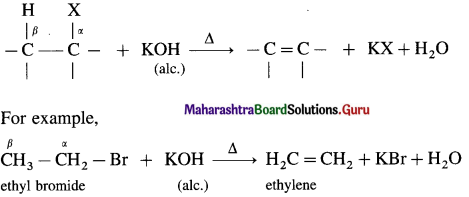
Tertiary butyl bromide when heated with alcoholic solution of potassium hydroxide forms isobutylene.

This reaction is called β-elimination (or 1,2-elimination) reaction as it involves elimination of halogen and a β-hydrogen atom.
As hydrogen and halogen is removed in this reaction it is also known as dehydrohalogenation reaction.
Question 60.
Describe the action of alcoholic potassium hydroxide (aic. KOH) on
(1) ethyl bromide
(2) n-propyl bromide
(3) isopropyl bromide
(4) tert-butyl chlorIde.
Answer:
(1) Ethyl bromide : When ethyl bromide (bromoethane) is heated with alcoholic potassium hydroxide (alcoholic alkali). ethene (gas) is formed by the dehydrobrominaion reaction.
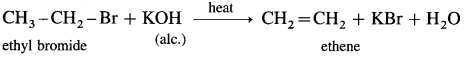
(2) n-PropI bromide : When n-propyl bromide is heated with alcoholic potassium hydroxide, propene is formed.

(3) Isopropvl bromide : When isopropyl bromide (2-bromopropane) is boiled with alcoholic potassium hydroxide, propcne is formed.
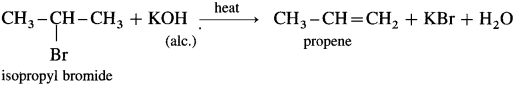
(4) Tert-hutyl chloride: When ten-butyl chloride (2-chloro-2-methyl propanc) is hcatcd with alcoholic KOH.

Question 61.
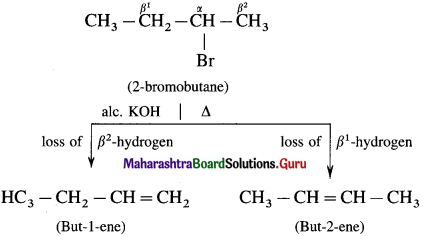
Describe the action of alc.KOH on 2-bromobutane.
When 2-bromobutane is boiled with alc.KOH on 2-bromobutane, a mixture of but-l-ene and but-2-ene is formed.
Answer:
![]()
Question 62.
Explain Saytzelf’s rule with suitable example.
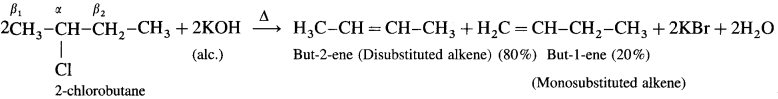
Answer:
Saytzcff’s rule : In dehydrohalogenation reaction the preferred product is that alkene which has the greater number of alkyl groups attached to the doubly bonded carbon atoms.
Hence the number of alkyl substituents on doubly bonded Carbon atoms increases, the stability of the alkene giving its major products.
Hence the increasing stability of alkenes is.

There are two types of fi hydrogens (β1 and β2) therefore two alkenes are expected.
Question 63.
What is a Grignard reagent ?
Answer:
Grignard reagent : An organometallic compound in which the divalent magnesium is directly linked to an alkyl group (R -) and a halogen atom (X), and has general formula R – Mg – X is called Grignard reagent. OR When alkyl halide is treated with magnesium in dry ether as solvent, it gives alkyl magnesium halide. It is known as Grignard reagent.
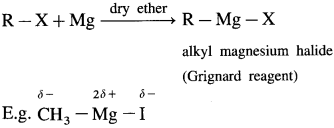
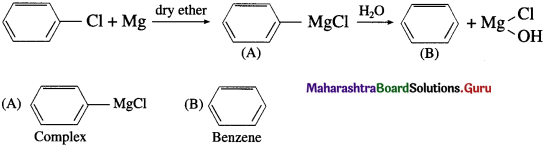
The carbon-magnesium bond is highly polar and magnesium-halogen bond is in ionic in nature. Grignard reagent is highly reactive. It is an important reagent and used in the preparation of a large number of organic compounds.
Question 64.
How is Grignard reagent prepared ?
Answer:
Grignard reagent is an alkyl magnesium halide, R – Mg – X obtained by the reaction of alkyl halide R – X with magnesium (Mg) in dry ether.
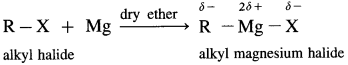
When an alkyl halide like CH3I is added from a dropping funnel to a flask containing pieces of pure Mg in pure and dry ether (diethyl ether) and a trace of iodine, Grignard reagent, CH3 – Mg – I is formed.
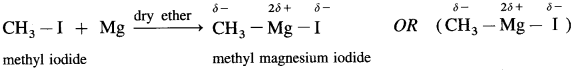
Ethyl iodide when treated with magnesium in presence of dry ether forms ethyl magnesium iodide.

Question 65.
Write a note on Grignard reagent.
Answer:
(1) Ethyl bromide : When ethyl bromide (bromoethane) is heated with alcoholic potassium hydroxide (alcoholic alkali). ethene (gas) is formed by the dehydrobrominaion reaction.

(2) n-PropI bromide : When n-propyl bromide is heated with alcoholic potassium hydroxide, propene is formed.

(3) Isopropvl bromide : When isopropyl bromide (2-bromopropane) is boiled with alcoholic potassium hydroxide, propcne is formed.

(4) Tert-hutyl chloride: When ten-butyl chloride (2-chloro-2-methyl propanc) is hcatcd with alcoholic KOH.


![]()
Question 66.
Describe the action of water on
(1) methyl magnesium iodide
(2) ethyl magnesium iodide.
Answer:
(1) Methyl magnesium iodide : When methyl magnesium iodide is treated with water, methane is obtained

(2) Ethyl magnesium iodide : When ethyl magnesium iodide is treated with water, ethane is obtained.

Question 67.
Describe the action of ammonia on
(1) ethyl magnesium bromide
(2) n-propyl magnesium chloride.
Answer:
(1) Ethyl magnesium bromide : When ethyl magnesium bromide is treated with ammonia, ethane is formed.

(2) n-Propyl magnesium chloride : When n-propyl magnesium chloride is treated with ammonia, propane is formed.

Question 68.
Explain Wurtz reaction. OR Explain the action of sodium with alkyl halides.
Answer:
(1) When an alkyl halide is treated with metallic sodium in dry ether, the corresponding higher alkane is formed. This is called Wurtz reaction or Wurtz coupling reaction.
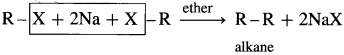
(2) In this reaction the alkyl radicals from two molecules of the reacting alkyl halide combine or couple to form the higher alkane.
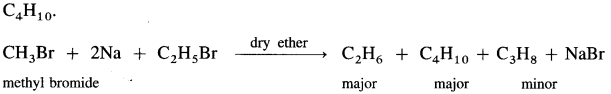
(3) Thus, methyl bromide reacts with sodium in ether to form ethane (C2H6), while ethyl bromide under the same conditions forms n-butane (C4H10).

(4) If a mixture of two different alkyl halides is treated with Na in dry ether, then a mixture of alkanes is obtained called self coupling products. For example, a mixture of CH3Br and C2H5Br gives propane along with C2H6 and C4H10.
Question 69.
Explain the reaction of haloarene with alkyl halide and sodium metal.
Write a note on Wurtz-Fittig reaction.
Answer:
When an alkyl halide and an aryl halide is treated with sodium metal in dry ether the corresponding alkylarene (alkyl benzene) is formed. The reaction is known as Wurtz-Fittig reaction. This reaction allows alkylation of alkyl halides.
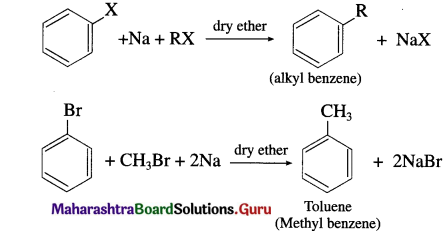
Question 70.
Describe the action of aryl halide on sodium metal.
Answer:
Aryl halide reacts with sodium metal in dry ether, biphenyl is formed. This reaction is known as Fittig reaction.

![]()
Question 71.
Identify the product A of following reaction.
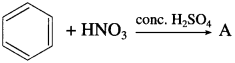
Answer:

Question 72.
Explain the following substitution reactions of chlorobenzene :
(1) Halogenation
(2) Nitration
(3) Sulphonation.
Answer:
(1) Halogenation : When chlorobenzene is reacted with chlorine in presence of anhydrous ferric chloride, a mixture of ortho and para-dichlorobenzene (major product) is formed.

(2) Nitration : When chlorobenzene is heated with nitrating mixture (cone, nitric acid -I- cone, sulphuric acid) a mixture of l-chloro-4-nitro benzene (major product) and l-chloro-2-nitrobenzene is formed.

(3) Sulphonation : When chlorobenzene is heated with concentrated sulphuric acid, a mixture of 4-chlorobenzene sulphonic acid (major product) and 2-chlorobenzene sulphonic acid is formed.

Question 73.
Describe the action of the following on chlorobenzene :
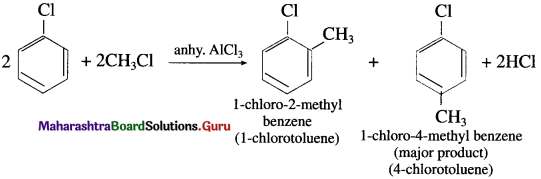
(1) Methyl chloride in the presence of anhydrous AICI3
(2) Acetyl chloride in the presence of anhydrous AICI3.
Answer:
(1) Methyl chloride in the presence of anhydrous AICI3 : When chlorobenzene is treated with methyl chloride in the presence of anhydrous AICI3, a mixture of l-chloro-4-methyl benzene (major product) and l-chloro-2-methyl benzene is formed. Since, the alkyl group is introduced in the benzene ring, the reaction is termed as Friedel Craft’s alkylation.
(2) Acetyl chloride in the presence of anhydrous AICI3 : When chlorobenzene is reacted with acetyl chloride in the presence of anhydrous AICI3, a mixture of 2-chloro acetophenone and 4-chloro acetophenone (major product) is formed. Since, the acetyl group is introduced in the benzene ring, the reaction is termed as Friedel Craft’s acylation.

![]()
Question 74.
Write a note on Friedel Craft’s reaction.
Answer:
(1) Methyl chloride in the presence of anhydrous AICI3 : When chlorobenzene is treated with methyl chloride in the presence of anhydrous AICI3, a mixture of l-chloro-4-methyl benzene (major product) and l-chloro-2-methyl benzene is formed. Since, the alkyl group is introduced in the benzene ring, the reaction is termed as Friedel Craft’s alkylation.

(2) Acetyl chloride in the presence of anhydrous AICI3 : When chlorobenzene is reacted with acetyl chloride in the presence of anhydrous AICI3, a mixture of 2-chloro acetophenone and 4-chloro acetophenone (major product) is formed. Since, the acetyl group is introduced in the benzene ring, the reaction is termed as Friedel Craft’s acylation.

Question 75.
Convert 1-chlorobutane into the following compounds :

Answer:
(1) 1-Chlorobutane to butan-l-ol :

(2) 1-Chlorobutane to 1-iodobutane :

(3) 1-Chlorobutane to n-butyl cyanide (CH3 – CH2 – CH2 – CH2 – CN) :
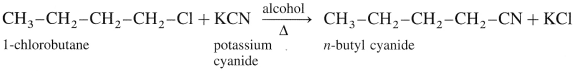
(4) l-Chlorobutane to 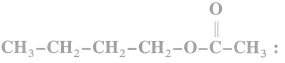

Question 76.
Predict the expected product of substitution reactions :
(1) Isobutyl chloride + sodium ethoxide.
Answer:

(2) n-butyl chloride + sodium.
Answer:

![]()
(3) 1-chloropropane + aq. potassium hydroxide.
Answer:
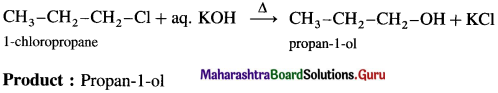
(4) Aniline + NaNO2/HCl.
Answer:

Question 77.
Write the products:

Question 78.
Identify A and B in the following :
![]()
Answer:

Answer:

![]()
Answer:
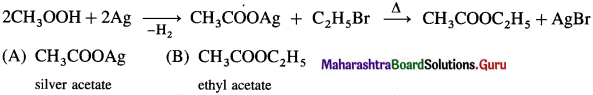
![]()
Answer:
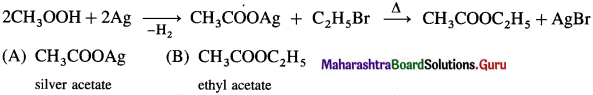
![]()
Answer:
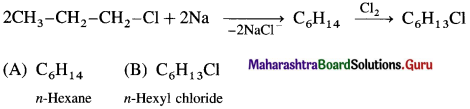
![]()
Answer:
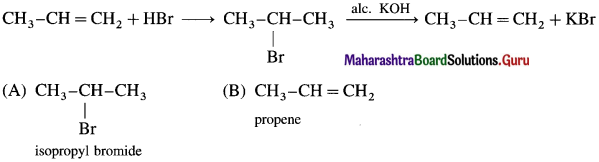
![]()
Question 79.
State the uses of the following compounds :
(1) Dichloromethane (CH2CI2)
(2) Trichloromethane or Chloroform (CHCI3)
(3) Tetrachloromethane or carbon tetrachloride (CCI4)
(4) Iodoform (CHI3)
(5) Freons
(6) DDT (p, p’-Dichlorodiphenyl trichloroethane).
Answer:
(1) Dichloromethane (CH2CI2) :
- Dichloromethane dissolves wide range of organic compounds, hence it is used as solvent for many chemical reactions.
- It is used as a solvent as a paint remover and degreaser.
- It is used as propellant in aerosols and as a fumigant pesticide for grains and strawberries.
- It is used to decaffinate tea or coffee.
(2) Trichloromethane or Chloroform (CHCI3) :
- Chloroform in the production of chlorofluoromethane, freon refrigerant R-22.
- It is used as solvent in pharmaceuticals, pesticides, gums, fats, resins and dye industry.
- It is a good source of dichlorocarbene species.
(3) Tetrachloromethane or carbon tetrachloride (CCI4) :
- Carbon tetrachloride is used in the manufacture of refrigerants.
- It is used as a dry cleaning agent and as a pesticide for stored grains.
- It is very useful solvent for oils, fats and resins. It serves as a source of chlorine.
(4) Iodoform (CHI3) :
- Iodoform is used as antiseptic, dressing of wounds and sores.
- On small scale it is used as disinfectant.
(5) Freons :
- Freons are widely used as propellants in aerosol, products of food, cosmetics and pharmaceutical industries.
- Freons containing bromine in their molecules are used as fire extinguishers.
- They are used in aerosol insecticides, solvent for cleaning clothes and metallic surfaces.
- It is used as foaming agents in the preparation of foamed plastics and in production of certain fluorocarbons.
- It is used as refrigerants and air conditioning purposes.
Question 80.
State the environmental effects of the following compounds :
(1) Dichloromethane (CH2CI2)
(2) Trichloromethane or chloroform (CHCI3)
(3) Tetrachloromethane or carbon tetrachloride (CCI4)
(4) Iodoform (CHI3)
(5) Freon.s (CCI2F2, CCI3F, CHCIF2)

Answer:
(1) Dichloromethane (CH2CI2) :
- Higher levels of dichloromethane in air causes nausea, numbness in fingers and toes, dizziness.
- Lower levels of dichloromethane causes impaired vision and hearing.
- Direct contact with eyes can damage cornea.
(2) Trichloromethane or chloroform (CHCI3) :
- When chloroform is exposed to air in the presence of sunlight, it slowly oxidised to phosgene, a poisonous compound, therefore it is stored in dark, amber coloured bottles.
- Chloroform vapour when inhaled for a short time causes dizziness, headache and fatigue and if inhaled for a long time affects central nervous system.
(3) Tetrachloromethane or carbon tetrachloride (CCI4) :
- Exposure to carbon tetrachloride causes eye irritation, damages nerve cells, vomiting sensation, dizziness, unconciousness or death. Long exposure to chloroform may affect liver.
- When mixed with air it causes depletion of the ozone layer, which affects human skin leading to cancer.
(4) Iodoform (CHI3) : Iodoform has a strong smell. It causes irritation to skin and eyes. It may cause respiratory irritation or breathing difficulty, dizziness, nausea, depression of central nervous system, visual disturbance.
(5) Freons (CCI2F2, CCI3F, CHCIF2) :
- Freon as refrigerant causes ozone depletion.
- Freons have low toxicity and low biological activity.
- Freons from propane group are more toxic in nature.
- Regular large inhalation of freon results in breathing problems, organ damage, loss of consciousness.
(6) DDT :
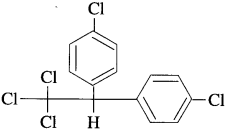
- DDT is not readily metabolised by animals.
- It is deposited and stored in fatty tissues.
- Exposure to high doses of DDT may cause vomiting, tremors or shakiness.
- Laboratory animal studies showed adverse effect of DDT on liver and reproduction.
- DDT is a pressistent organic pollutant, readily absorbed in soils and tends to accumulate in the ecosystem.
- When dissolved in oil or other lipid, it is readily absorbed by the skin. It is resistant to metabolism.
- There is a ban on use of DDT due to all these adverse effects.
![]()
Question 81.
What is the chemical name of freon?
Answer:
The chemical name of freon is Dichlorodifluoromethane.
Question 82.
What is the chemical name of DDT ?
Answer: The chemical name of DDT is p, p’-Dichlorodiphenyltrichloroethane.
Activity :
(1) Collect detailed information about Freons and their uses.
(2) Collect information about DDT as a persistent pesticide.
Reference books :
(1) Organic chemistry by Morrison, Boyd, Bhattacharjee, 7th edition, Pearson.
(2) Organic chemistry by Finar, Vol 1, 6th edition, Pearson
Multiple Choise Questions
Question 83.
Select and write the most appropriate answer from the given alternatives for each sub-question :
Question 1.

Answer:
(b) CH3 – CH2 – CH2 – I
Question 2.
The rate of SN2 reaction depends on the concentra¬tion of
(a) only the substrate
(b) only the reagent
(c) both the substrate and the reagent
(d) neither the substrate nor the reagent
Answer:
(c) both the substrate and the reagent
Question 3.
In SN2 reaction, the hydrolysis of alkyl halide shows
(a) the retention of configuration
(b) the inversion of configuration
(c) both retention and inversion of configuration
(d) no change in the configuration
Answer:
(b) the inversion of configuration
Question 4.
The one step exothermic reaction is
(a) SN1
(b) SN2
(C) SN
(d) S2N
Answer:
(b) SN2
Question 5.
Which of the following is correct about SN2 mechanism?
(a) Two step reaction
(b) Complete inversion of configuration
(c) Formation of carbonium ion
(d) Favoured by polar solvent
Answer:
(b) Complete inversion of configuration
Question 6.
Which of the following is not a nucleophile?
(a) Ammonia
(b) Ammonium ion
(c) Primary amine
(d) Secondary amine
Answer:
(d) Secondary amine
![]()
Question 7.
Which of the following undergoes nucleophilic substitution exclusively by SN2 mechanism ?
(a) ethyl chloride
(b) isopropyl chloride
(c) chlorobenzene
(d) benzyl chloride
Answer:
(d) benzyl chloride
Question 8.
Which of the following is most reactive towards nucleophilic substitution reaction ?
(a) CH2 = CH – CI
(b) CH3CH = CHCI
(c) C6H5CI
(d) CICH2 – CH = CH2
Answer:
(d) CICH2 – CH = CH2
Question 9.
The stability order of carbocation is
(a) 2° > 3° > 1°
(b) 3° > 2° > 1°
(c) 3° > 1° > 2°
(d) 1° > 3° > 2°
Answer:
(b) 3° > 2° > 1°
Question 10.

(a) ethane
(b) propane
(c) n-butane
(d) n-pentane
Answer:
(c) n-butane
Question 11.
Which of the following characteristic properties of the enantiomers is correct?
(a) The enantiomers possess same physical and chemical properties
(b) The enantiomers are optically active compounds
(c) The enantiomers have different optical rotations
(d) All of these
Answer:
(d) All of these
Question 12.
The optically inactive compound is
(a) glucose
(b) lactic acid
(c) isopropyl alcohol
(d) 2-bromo butane
Answer:
(c) isopropyl alcohol
Question 13.
A compound with the molecular formula CH2OH(CHOH)3CH2OH has optically active forms
(a) 3
(b) 4
(c) 6
(d) 8
Answer:
(d) 8
![]()
Question 14.
A racemic mixture consists of
(a) equal amount of d and l isomers
(b) unequal amounts of d and / isomers
(c) unknown amounts of d and / isomers
(d) only d isomers
Answer:
(a) equal amount of d and l isomers
Question 15.
Which of the following compounds is not optically active ?
(a) Lactic acid
(b) Secondary butyl chloride
(c) n-propyl iodide
(d) Glucose
Answer:
(c) n-propyl iodide
Question 16.
Which of the following compounds shows optical activity ?
(a) n-butyl chloride
(b) isobutyl chloride
(c) sec-butyl chloride
(d) t-butyl chloride
Answer:
(c) sec-butyl chloride
Question 17.
The major product of the following reaction is

Answer:
(c)
Question 18.
The above reaction is known as

(a) Wurtz-Fittig reaction
(b) Friedel Craft’s reaction
(c) Sandmeyer’s reaction
(d) Swarts reaction
Answer:
(b) Friedel Craft’s reaction
![]()
Question 19.
Iodoform is used as
(a) an anaesthetic
(b) an antiseptic
(c) an analgesic
(d) an antibiotic
Answer:
(b) an antiseptic
Question 20.
p, p’-dichlorodiphenyl trichloroethane is used as
(a) insecticide
(b) anaesthetic
(c) antiseptic
(d) refrigerant
Answer:
(a) insecticide
Question 21.
The order of reactivity in nucleophilic substitution reaction is
(a) CH3F < CH3C1 < CH3I < CH3Br
(b) CH3F < CH3C1 < CH3Br < CH3I
(c) CH3F < CH3Br < CH3C1 < CH3I
(d) CH3I < CH3Br < CH3C1 < CH3F
Answer:
(b) CH3F < CH3C1 < CH3Br < CH3I
Question 22.
Racemate is
(a) optically active
(b) optically dextro rotatory
(c) optically inactive
(d) optically laevorotatory
Answer:
(c) optically inactive
Question 23.
The number of asymmetric carbon atoms in glucose are
(a) 2
(b) 3
(c) 4
(d) 5
Answer:
(c) 4
Question 24.
The geometry of carbon lum ion is
(a) Tetrahedral
(b) planar
(c) linear
(d) pyramidal
Answer:
(b) planar
Question 25.
In its nucleophilic substitution reaction, aryl halide resembles
(a) Vinyl chloride
(b) allyl chloride
(e) Benzyl chloride
(d) ethyl chloride
Answer:
(a) Vinyl chloride
Question 26.
The weakest C-Cl bond is present in
Answer:
(d)
![]()
Question 27.
Which alkyl halide among the following com¬pounds has the highest boiling point ?
(a) (CH3)3CCI
(b) CH3CH2CH2CH2CI
(c) CH3CH2CH2C1
(d) CH3CH(CH3)CH2CI
Answer:
(b) CH3CH2CH2CH2CI
Question 28.
It is difficult to break C-Cl bond in CH2 = CH – CI due to
(a) Hyper conjugation
(b) Resonance
(c) Electromeric effect
(d) Inductive effect
Answer:
(b) Resonance
Question 29.
Which one of the following when heated with metallic sodium will not give the corresponding alkane ?

Answer:
(c)
Question 30.
The most reactive alkyl halide towards SN2 reac¬tion is
(a) CH3X
(b) R3CX
(C) R2CHX
(d) RCH2X
Answer:
(a) CH3X
Question 31.
The number of electrons surrounding the carbon- ium ion is
(a) 6
(b) 8
(c) 10
(d) 7
Answer:
(a) 6
Question 32.
The lowest stability of carbocation among the compounds

Answer:
(a)
Question 33.
Carbon atom in methyl carbocation contains how many pairs of electrons?
(a) 8
(b) 4
(c) 3
(d) 5
Answer:
(b) 4
![]()
Question 34.
The optically inactive compound is
(a) Glucose
(b) Lactic acid
(c) 2-Chlorobutane
(d) 2-Chloropropane
Answer:
(d) 2-Chloropropane
Question 35.
The hydrogen halide which does not obey Markownikv rule in presence of peroxide is
(a) HC1
(b) HBr
(c) HF
(d) HI
Answer:
(b) HBr
Question 36.
Which one of the following is NOT used to prepare alkyl halide from an alcohol ?
(a) SOCl2
(b) PC13
(c) HC1 + ZnCl2
(d) NaCl
Answer:
(d) NaCl
Question 37.
The total number of electrons present in the central carbon atom of a free radical is
(a) 7
(b) 8
(c) 9
(d) 6
Answer:
(a) 7
Question 38.
In which of the following pairs both are nucleophiles ?
(a) BF3, AICI3
(b) NO+2, Cl–
(c) CN–, NH3
(d) Br+, BC13
Answer:
(c) CN–, NH3
Question 39.
Which one of the following alkane is NOT formed in Wurtz reaction ?
(a) Methane
(b) Ethane
(c) Propane
(d) Butane
Answer:
(a) Methane
Question 40.
Which of the following groups has highest priority according to R, S convention?
(a) CH2OH
(b) COOH
(c) COCH3
(d) COOCH3
Answer:
(d) COOCH3
Question 41.
The halogen atom in aryl halides is
(a) o- and p-di reefing
(b) m-directing
(c) o, m and p-di reefing
(d) only m-directing
Answer:
(a) o- and p-di reefing
![]()
Question 42.
Chlorobenzene can be obtained by benzene diazonium chloride by
(a) Friedel Craft’s reaction
(b) Wurtz reaction
(c) Gatterman’s reaction
(d) Fittig reaction
Answer:
(c) Gatterman’s reaction
Question 43.
Which of the following carbocations is least stable ?


Answer:
(c)
Question 44.
But-l-ene on reaction with HCI in the presence of sodium peroxide yields
(a) n-butyl chloride
(b) isobutyl chloride
(c) secondary butyl chloride
(d) tertiary butyl chloride
Answer:
(c) secondary butyl chloride
Question 45.
Carbon tetrachloride is used as
(a) anaesthetic
(b) antiseptic
(c) dry cleaning agent
(d) fire extinguisher
Answer:
(c) dry cleaning agent
Question 46.
Identify the product D in the following sequence of reactions :

(a) 2, 2-dimethyl butane
(b) 2, 3-dimethyl butane
(C) hexane
(d) 2, 4-dimethylpentane
Answer:
(b) 2, 3-dimethyl butane
Question 47.
The preparation of alkyl fluoride from alkyl chlor ide, in presence of metallic fluorides is known as
(a) Williamson’s reaction
(b) Finkeistein reaction
(c) Swarts reaction
(d) Wurlz reaction
Answer:
(c) Swarts reaction
![]()
Question 48.
UPAC name of the following compound is
![]()
(a) 3-Bromo-3, 4-dimetbyiheptane
(b) 3,4-dimethyl-3-bromoheptane
(c) 5-Bromo-4,5-dimethylheptane
(d) 4,5-dimethyl-5-bromoheptane
Answer:
(a) 3-Bromo-3, 4-dimetbyiheptane