Balbharti Maharashtra State Board 11th Chemistry Important Questions Chapter 5 Chemical Bonding Important Questions and Answers.
Maharashtra State Board 11th Chemistry Important Questions Chapter 5 Chemical Bonding
Question 1.
Explain the electronic theory of valence.
Answer:
Electronic theory of valence:
- The electronic theory of valence was proposed by Kossel and Lewis in 1916.
- They gave a logical explanation of valence which was based on the inertness of noble gases (that is, the octet rule developed by Lewis).
- According to Lewis, the atom can be pictured in terms of a positively charged ‘kernel’ (the nucleus plus inner electrons) and outer shell that can accommodate a maximum of eight electrons. This octet of electrons represents a stable electronic arrangement.
- Thus, according to this theory, during the formation of a chemical bond, each atom loses, gains or shares outer electrons so that it achieves stable octet.
- The formation of NaCl involves transfer of one electron from sodium (Na) to chlorine (Cl). Na+ and Cl– ions are formed which are held together by chemical bond. The formation of H2, F2, Cl2, HCl, etc., involves sharing of a pair of electrons between the atoms. In both cases, each atom attains a stable outer octet of electrons.
Question 2.
Give the significance of octet rule. Explain why this rule is not valid for H and Li atoms.
Answer:
i. Significance: Octet rule is found to be very useful:
- in explaining the normal valence of elements
- in the study of the chemical combination of atoms leading to the formation of molecules.
ii. Octet rule is not valid for H and Li atoms. According to octet rule, during the formation of a chemical bond, each atom loses, gains or shares electrons so that it achieves stable octet (eight electrons in the valence shell). However, H and Li atoms tend to have only two electrons in their valence shell similar to that of Helium (1s2), which called duplet. Hence, octet rule is not valid for H and Li atoms.
Question 3.
Define ionic bond.
Answer:
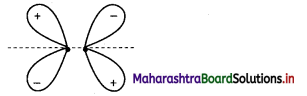
The bond formed by complete transfer of one or more electrons from an electropositive atom to an electronegative atom, leading to formation of ions which are held together by electrostatic attraction is called ionic bond or electrovalent bond.
![]()
Question 4.
Explain the formation of ionic bond in sodium chloride (NaCl).
Answer:
Formation of sodium chloride (NaCl):
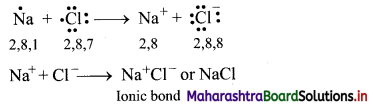
i. The electronic configurations of sodium and chlorine are:
Na (Z = 11): 1s2 2s2 2p6 3s1 or (2, 8, 1)
Cl (Z = 17): 1s2 2s2 2p6 3s2 3p5 or (2, 8, 7)
ii. Sodium has one electron in its valence shell. It has tendency to lose one electron to acquire the electronic configuration of the nearest inert gas, neon (2, 8).
iii. Chlorine has seven electrons in its valence shell. It has tendency to gain one electron and thereby acquire the electronic configuration of the nearest inert gas, argon (2, 8, 8).
iv. During the combination of sodium and chlorine atoms, the sodium atom transfers its valence electron to the chlorine atom.
v. Sodium atom changes into Na+ ion while the chlorine atom changes into Cl– ion. The two ions are held together by strong electrostatic force of attraction.
vi. The formation of ionic bond between Na and Cl can be shown as follows:
Question 5.
Explain the formation of ionic bonds in calcium chloride (CaCl2).
Answer:
Formation of calcium chloride (CaCl2):
i. The electronic configurations of calcium and chlorine are:
Na (Z = 11): 1s2 2s2 2p6 3s2 3p6 4s2 or (2, 8, 8, 2)
Cl (Z = 17): 1s2 2s2 2p6 3s2 3p5 or (2, 8, 7)
ii. Calcium has two electrons in its valence shell. It has tendency to lose two electrons to acquire the electronic configuration of the nearest inert gas, argon (2, 8, 8).
iii. Chlorine has seven electrons in its valence shell. It has tendency to gain one electron and thereby acquire the electronic configuration of the nearest inert gas, argon (2, 8, 8).
iv. During the combination of calcium and chlorine atoms, the calcium atom transfers its valence electrons to two chlorine atoms.
v. Calcium atom changes into Ca2+ ion while the two chlorine atoms change into two Cl– ions. These ions are held together by strong electrostatic force of attraction.
vi. The formation of ionic bond(s) between Ca and Cl can be shown as follows:
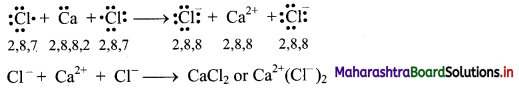
Question 6.
What are ionic solids?
Answer:
Ionic solids are solids which contain cations and anions held together by ionic bonds.
e.g. Sodium chloride (NaCl), Calcium chloride (CaCl2)
Question 7.
Define lattice enthalpy.
Answer:
Lattice enthalpy of an ionic solid is defined as the energy required to completely separate one mole of solid ionic compound into the gaseous components.
Note: Lattice enthalpy values of some ionic compounds:
| Compound | Lattice enthalpy kJ mol–1 |
| LiCl | 853 |
| NaCl | 788 |
| BeF2 | 3020 |
| CaCl2 | 2258 |
| AlCl3 | 5492 |
Question 8.
Arrange NaCl, CaCl2 and AlCl3 in increasing order of lattice enthalpy (positive value). Justify your answer.
Answer:
Compounds having cations with higher charge have large lattice enthalpy (higher positive value) than compounds having cations with lower charge.
Hence, the correct order is NaCl < CaCl2 < AlCl3.
![]()
Question 9.
Lattice enthalpy of LiF is more than that of NaF. Explain.
Answer:
As the size of the cation decrease, lattice enthalpy increases. Li+ ion is smaller than Na+ ion. Hence, lattice enthalpy of LiF is more than that of NaF.
Question 10.
Define covalent bond.
Answer:
The attractive force which exists due to the mutual sharing of electrons between the two atoms of similar electronegativity or having small difference in electronegativities is called a covalent bond.
Question 11.
Explain the formation of covalent bond in H2 molecule.
Answer:
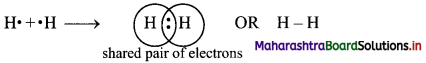
Formation of H2 molecule:
i. The electronic configuration of H atom is 1s1.
ii. It needs one more electron to complete its valence shell.
iii. When two hydrogen atoms approach each other at a certain internuclear distance, they share their valence electrons.
iv. The shared pair of electrons belongs equally to both the hydrogen atoms. The two atoms are said to be linked by a single covalent bond and a H2 molecule is formed.
Question 12.
Explain the formation of covalent bond in Cl2 molecule.
Answer:
Formation of Cl2 molecule:
i. The electronic configuration of Cl atom is [Ne] 3s2 3p5.
ii. It needs one more electron to complete its valence shell.
iii. When two chlorine atoms approach each other at a certain internuclear distance, they share their valence electrons. In the process, both the atoms attain the valence shell of octet of nearest noble gas, argon.
iv. The shared pair of electrons belongs equally to both the chlorine atoms. The two atoms are said to be linked by a single covalent bond and a Cl2 molecule is formed.
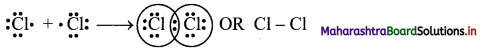
Question 13.
What are the important features of covalent bond?
Answer:
- Each covalent bond is formed as a result of sharing of electron pair between the two atoms.
- When a covalent bond is formed, each combining atom contributes one electron to the shared pair.
- The combining atoms attain the outer shell noble gas configuration as a result of the sharing of electrons.
![]()
Question 14.
Explain the types of covalent bond with suitable examples.
Answer:
The three types of covalent bonds are as follows.
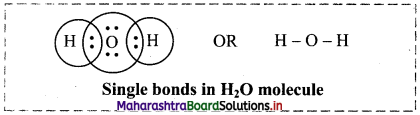
i. Single bond: When two combining atoms share one electron pair, the covalent bond between them is called single bond.
Single bond is observed in number of molecules.
e.g. H2, Cl2, water molecule, etc.
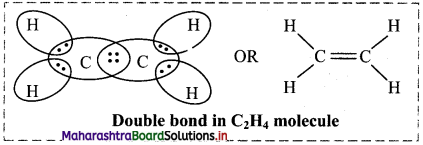
ii. Double bond: When two combining atoms share two pairs of electrons, the covalent bond between them is called a double bond, e.g. Double bond is present in C2H4 molecule
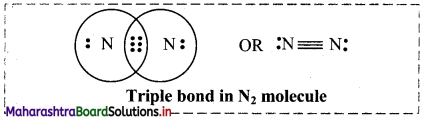
iii. Triple bond: When two combining atoms share three pairs of electrons, the covalent bond between them is called a triple bond, e.g. Triple bond is present in N2 molecule.
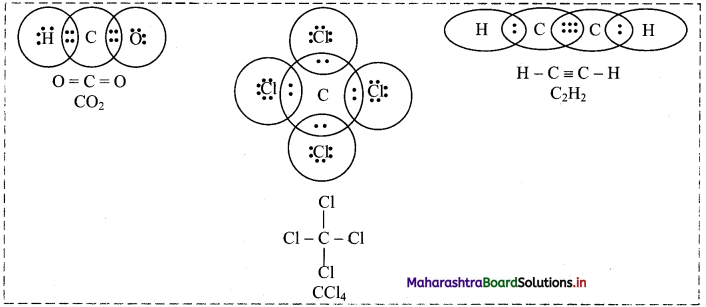
Note: Formation of covalent bonds in CO2, CCl4 and C2H2:
Question 15.
Distinguish between ionic bond and covalent bond.
Answer:
Ionic bond:
- It is formed by the transfer of electrons from one atom to another.
- It is formed by the transfer of electrons from one atom to another.
- In this, oppositely charged ions are formed.
- There are no multiple ionic bonds.
- This bond usually exists between metal and non-metal atoms.
- e.g. NaCl, CaCl2
Covalent bond:
- It is formed by sharing of electrons.
- Atoms are held together due to shared pair of electrons.
- In this, oppositely charged ions are not formed.
- Covalent bonds may be single or double or triple bonds.
- This bond usually exists between non-metal atoms.
- e.g. H2, Cl2
Question 16.
What are the steps to write Lewis dot structure?
Answer:
Steps to write Lewis dot structures:
- Add the total number of valence electrons of combining atoms in the molecule.
- In anions, add one electron for each negative charge.
- In cations, subtract one electron from valence electrons for each positive charge.
- Write skeletal structure of the molecule to show the atoms and number of valence electrons forming the single bond between the atoms.
- Add remaining electron pairs to complete the octet of each atom.
- If octet is not complete form multiple bonds between the atoms such that octet of each atom is complete.
- In polyatomic atoms and ions, the least electronegative atom is the central atom.
e.g. In \(\mathrm{SO}_{4}^{2-}\) ion, ‘S’ is the central atom and in \(\mathrm{NO}_{3}^{-}\), ‘N’ is the central atom. - After writing the number of electrons as shared pairs forming single bonds, the remaining electron pairs are used either for multiple bonds or they remain as lone pairs.
Question 17.
Write the Lewis structure of nitrite ion, \(\mathrm{NO}_{2}^{-}\).
Answer:
Step I: Count the total number of valence electrons of nitrogen atom, oxygen atom and one electron of additional negative charge.
Valence shell configuration of nitrogen and oxygen are:
N ⇒ (2s2 2p3), O ⇒ (2s2 2p4)
The total electrons available are:
5 + (2 × 6) + 1 = 18 electrons
Step II: The skeletal structure of \(\mathrm{NO}_{2}^{-}\) is written as O N O
Step III: Draw a single bond i.e., one shared electron pair between the nitrogen and each oxygen atoms. Then distribute the remaining electrons to achieve noble gas configuration for each atom. This does not complete the octet of nitrogen.
![]()
Hence, there is a multiple bond between nitrogen and one of the oxygen atoms (a double bond). The remaining two electrons constitute a lone pair on nitrogen.
Following are Lewis dot structures of \(\mathrm{NO}_{2}^{-}\).
![]()
![]()
Question 18.
Write the Lewis structure of CO molecule.
Answer:
Step I: Count number of electrons of carbon and oxygen atoms. The valence shell configuration of carbon and oxygen atoms are: 2s2 2p2 and 2s2 2p4, respectively. The valence electrons available are:
4 + 6=10
Step II: The skeletal structure of CO is written as
C O
Step III: Draw a single bond (One shared electron pair) between C and O and complete the octet on O. The remaining two electrons is a lone pair on C.
![]()
The octet on carbon is not complete. Hence, there is a multiple bond between C and O (a triple bond between C and O atom). This satisfies the octet rule for carbon and oxygen atoms.
The Lewis structure of CO molecule is:
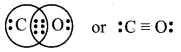
Question 19.
Explain the term formal charge.
Answer:
i. Formal charge is the charge assigned to an atom in a molecule, assuming that all electrons are shared equally between atoms, regardless of their relative electronegativities.
ii. While determining the best Lewis structure per molecule, the structure is chosen such that the formal charge is as close to zero as possible.
iii. The structure having the lowest formal charge has the lowest energy.
iv. Formal charge is assigned to an atom based on electron dot structures of the molecule/ion.
v. Formal charge on an atom in a Lewis structure of a polyatomic species can be determined using the following formula:
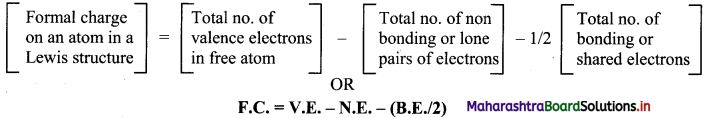
Question 20.
Explain the calculation of the formal charge on oxygen atoms in case of O3 (ozone) molecule.
Answer:
i. Lewis dot structure of O3 (ozone) molecule is:

Three oxygen atoms are present in the O3 molecule and are labelled as 1, 2 and 3.
ii. Formal charges on oxygen atoms labelled as 1, 2, 3 are calculated as shown below:
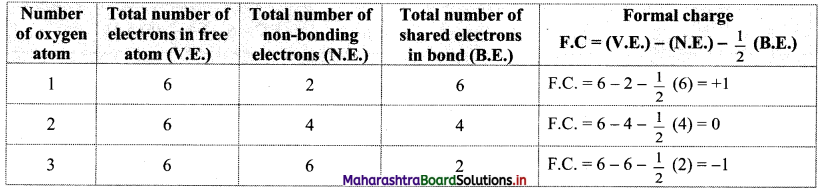
iii. On the basis of the formal charge values, O3 is shown as

[Note: Indicating the charges on the atoms in the Lewis structure helps in keeping track of the valence electrons in the molecule. Formal charges help in the selection of the lowest energy structure from a number of possible Lewis structures for a given species.]
Question 21.
CO2 can be represented by following three structures:
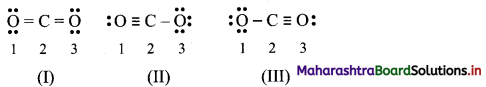
Calculate the formal charge on each atom in all the three structures of CO2 molecule. Identify the structure with lowest energy.
Answer: Formal charges on atoms labelled as 1, 2, 3 are calculated as shown below:
Structure (I):
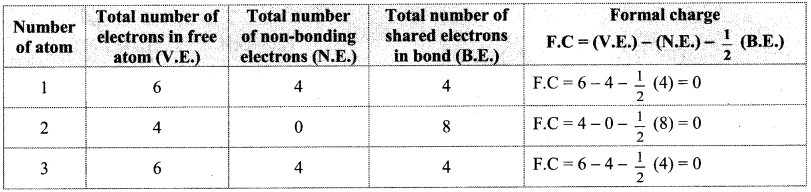
Structure (II):
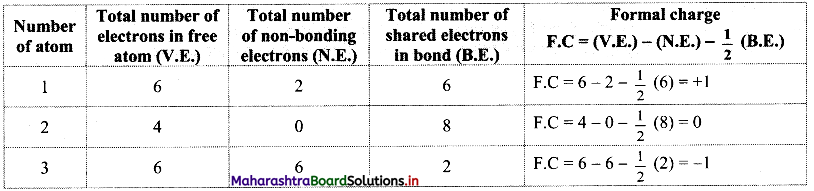
Structure (III):
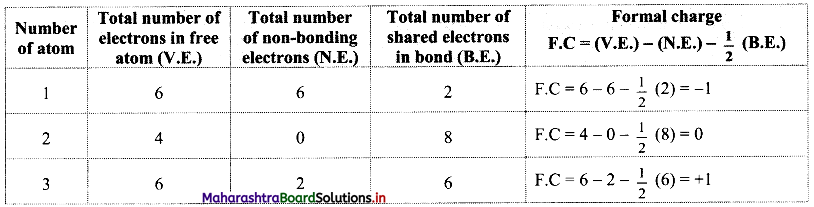
While determining the best Lewis structure per molecule, the structure is chosen such that the formal charge is as close to zero as possible. The structure having the lowest formal charge has the lowest energy.
In structure (I), the formal charge on each atom is 0 while in structures (II) and (III) formal charge on carbon is 0 while oxygens have formal charge -1 or +1. Hence, the possible structure with the lowest energy will be structure (I). Thus, formal charges help in the selection of the lowest energy structure from a number of possible Lewis structures for a given species.
Question 22.
Find out the formal charges on S, C and N.
(S = C = N)– ; (S – C ≡ N)– ; (S ≡ C – N)–
Answer:
Step I:
Write Lewis dot diagrams for the structures:
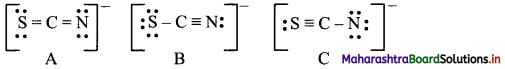
Step II:
Assign formal charges for all the atoms:
F.C. = V.E. – N.E. – 1/2 (B.E.)
Structure A:
Formal charge on S = 6 – 4 – 1/2(4) = 0
Formal charge on C = 4 – 0 – 1/2 (8) = 0
Formal charge on N = 5 – 4 – 1/2 (4) = -1
Structure B:
Formal charge on S = 6 – 6 – 1/2(2) = -1
Formal charge on C = 4 – 0 – 1/2 (8) = 0
Formal charge on N = 5 – 2 – 1/2 (6) = 0
Structure C:
Formal charge on S = 6 – 2 – 1/2(6) = +1
Formal charge on C = 4 – 0 – 1/2(8) = 0
Formal charge on N = 5 – 6 – 1/2(2) = -2
![]()
Question 23.
Give the limitations of octet rule:
Answer:
Limitations of octet rule:
i. Octet rule does not explain stability of some molecules.
The octet rule is based on the inert behaviour of noble gases, which have their octet complete i.e., have eight electrons in their valence shell. It is very useful to explain the structures and stability of organic molecules. However, there are many molecules whose existence cannot be explained by the octet theory. The central atoms in these molecules does not have eight electrons in their valence shell, and yet they are stable.
Such molecules can be categorized as having:
a. Incomplete octet
b. Expanded octet
c. Odd electrons
a. Molecules with incomplete octet: e.g. BF3, BeCl2, LiCl
In these covalent molecules, the atoms B, Be and Li have less than eight electrons in their valence shell but these molecules are stable.
Li in LiCl has only two electrons, Be in BeCl2 has four electrons while B in BF3 has six electrons in the valence shell.
b. Molecules with expanded octet: Some molecules like SF6, PCl5, H2SO4 have more than eight electrons around the central atom.
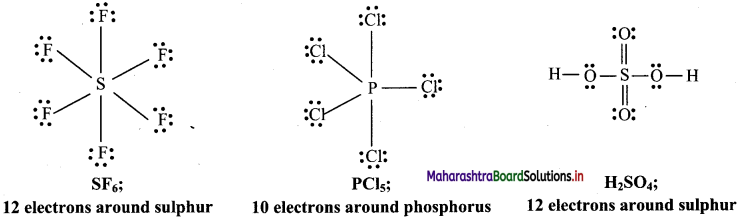
c. Odd electron molecules:
Some molecules like NO (nitric oxide) and NO2 (nitrogen dioxide) do not obey the octet rule. These molecules, have odd number of valence electrons.
![]()
ii. The observed shape and geometry of a molecule, cannot be explained, by the octet rule.
iii. Octet rule fails to explain the difference in energies of molecules, though all the covalent bonds are formed in an identical manner, that is, by sharing a pair of electrons. The rule fails to explain the differences in reactivities of different molecules.
Note: Sulphur also forms many compounds in which octet rule is obeyed. For example, in sulphur dichloride, the sulphur atom has eight electrons around it.

Question 24.
State the basic idea on which VSEPR theory was proposed by Sidgwick and Powell.
Answer:
Valence Shell Electron Pair Repulsion (VSEPR) theory is based on the basic idea that the electron pairs on the atoms shown in the Lewis diagram repel each other. In the real molecule, they arrange themselves in such a way that there is minimum repulsion between them.
Question 25.
Give the rules of VSEPR Theory.
Answer:
Rules of VSEPR Theory:
i. Electron pairs arrange themselves in such a way that repulsion between them is minimum.
ii. The molecule acquires minimum energy and maximum stability.
iii. Lone pair of electrons also contribute in determining the shape of the molecule.
iv. Repulsion of other electron pairs by the lone pair (L.P.) stronger than that of bonding pair (B.P.).
Trend for repulsion between electron pair is as follows:
L.P. – L.P. > L.P. – B.P. > B.P. – B.P.
Lone pair-Lone pair repulsion is maximum because this electron pair is under the influence of only one nucleus while the bonded pair is shared between two nuclei.
Thus, the number of lone pair and bonded pair of electrons decide the shape of the molecules. Molecules having no lone pair of electrons have a regular geometry.
Note:
i. Electron pair geometry: The arrangement of electrons around the central atom is called as electron pair geometry. These electron pairs may be shared in a covalent bond or they may be lone pairs.
ii. Geometry of some molecules (having no lone pair of electrons):
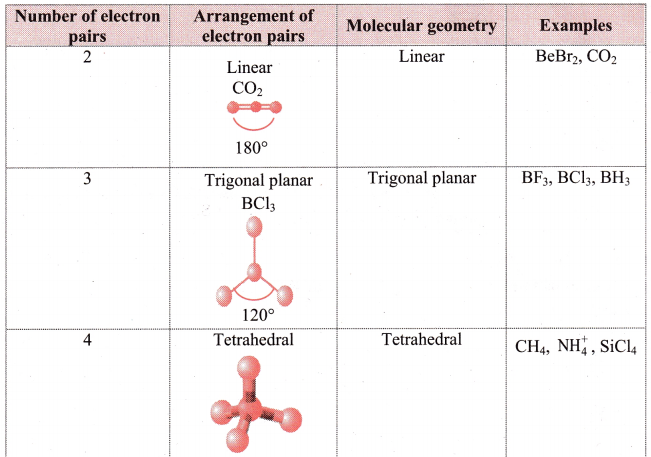
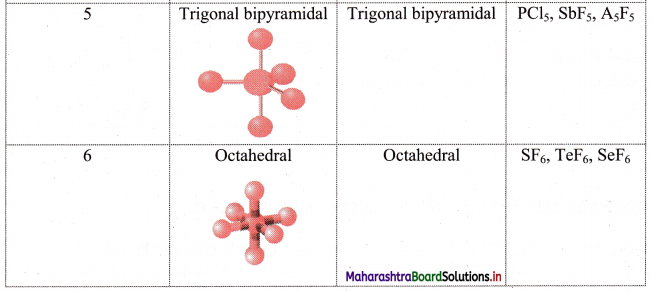
iii. Geometry of some molecules (having one or more lone pairs of electrons):
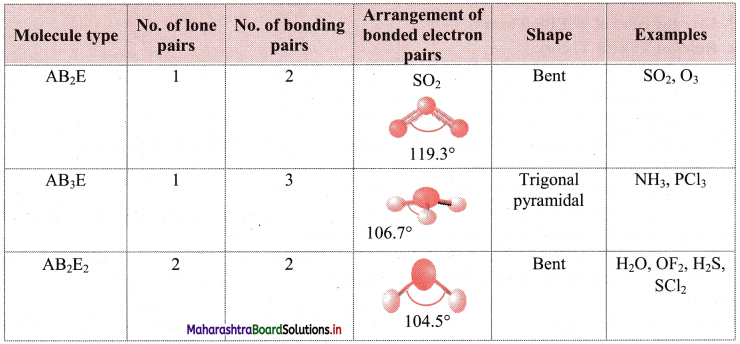
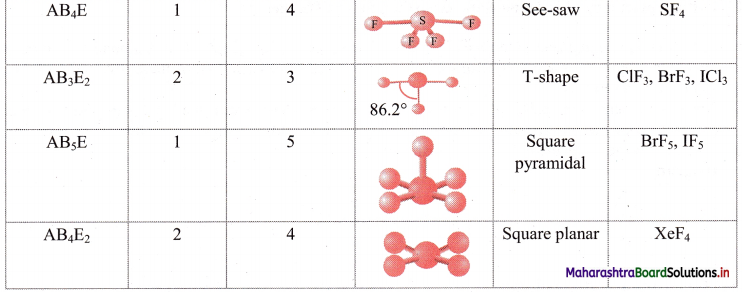
Question 26.
Match the following:
| Molecule | Shape | ||
| i. | SbF5 | a. | Trigonal bipyramidal |
| ii. | SO2 | b. | Bent |
| iii. | SF4 | c. | Square pyramidal |
| iv. | IF5 | d. | See-saw |
Answer:
i – a,
ii – b,
iii – d,
iv – c
![]()
Question 27.
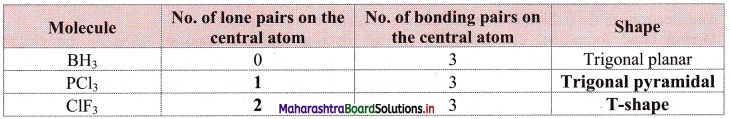
Complete the following table:
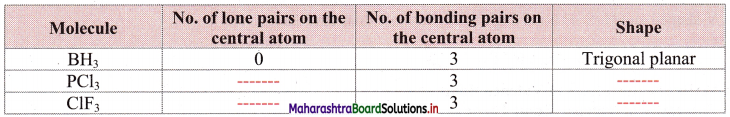
Answer:
Question 28.
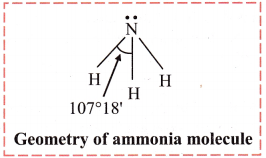
Explain geometry of NH3 molecule according to VSEPR theory.
Answer:
- In NH3 molecule, the central atom nitrogen has five electrons in its valence shell. On bond formation with three hydrogen atoms, there are 8 electrons in the valence shell of nitrogen. Out of these, three pairs are bond pairs (N – H covalent bonds) and one forms lone pair. The expected geometry is tetrahedral and bond angle is 109° 28′.
- There are two types of repulsions between the electron pairs: Lone pair – bond pair and bond pair – bond pair
- The lone pair – bond pair repulsions are stronger and the bonded pairs are pushed inwards. Thus, reducing the bond angle to 107°18′ and shape of the molecule becomes trigonal pyramidal.
Diagram:
Question 29.
Explain geometry of H2O molecule according to VSEPR theory.
Answer:
- In H2O molecule, the central atom oxygen has six electrons in its valence shell. On bond formation with two hydrogen atoms, there are 8 electrons in the valence shell of oxygen. Out of these, two pairs are bond pairs and two are lone pairs.
- Due to lone pair – lone pair repulsion, the lone pairs are pushed towards the bond pairs and bond pair – lone pair repulsions become stronger thereby reducing the H-O-H bond angle from 109° 28′ to 104° 35′ and the geometry of the molecule becomes angular (bent).
Diagram:

Question 30.
Write the postulates of Valence Bond Theory.
Answer:
Postulates of Valence Bond Theory:
- A covalent bond is formed when the half-filled valence orbital of one atom overlaps with the half-filled valence orbital of another atom.
- The electrons in the half-filled valence orbitals must have opposite spins.
- During bond formation, the half-filled orbitals overlap and the opposite spins of the electrons get neutralized. The increased electron density decreases the nuclear repulsion and energy is released during overlapping of the orbitals.
- Greater the extent of overlap, stronger is the bond formed. However, complete overlap of orbitals does not take place due to intemuclear repulsions.
- If an atom possesses more than one unpaired-electrons, then it can form more than one bond. So, number of bonds formed will be equal to the number of half-filled orbitals in the valence shell i.e., number of unpaired electrons.
- The distance at which the attractive and repulsive forces balance each other is the equilibrium distance between the nuclei of the bonded atoms. At this distance, the total energy of the two bonded atoms is minimum and stability of the molecule is maximum.
- Electrons which are paired in the valence shell cannot participate in bond formation. However, in an atom if there is one or more vacant orbital present then these electrons can unpair and participate in bond formation provided the energies of the filled and vacant orbitals differ slightly from each other.
- During bond formation, the ‘s’ orbital which is spherical can overlap in any direction. The ‘p’ orbitals can overlap only in the x, y or z directions. Similarly, ‘d’ and ‘f orbitals are oriented in certain directions in space and overlap only in these directions. Thus, the covalent bond is directional in nature.
Note: In order to explain the covalent bonding, Heitler and London developed the valence bond theory on the basis of wave mechanics. This theory was further extended by Pauling and Slater.
![]()
Question 31.
Explain the formation of hydrogen molecule with the help of potential energy curve.
OR
Explain the formation of H2 on the basis of VBT.
Answer:
Formation of H2 on the basis of VBT:
- Hydrogen atom has electronic configuration 1s1. It contains one unpaired electron in its valence shell.
- When the two hydrogen atoms containing unpaired electrons with opposite spins are separated by a large distance, they can neither attract nor repel each other (there are no interactions between them). The energy of the system is the sum of the potential energies of the two atoms which is arbitrarily taken as zero.
- When the two atoms approach each other, attractive and repulsive forces begin to operate on them. Experimentally, it has been found that during formation of hydrogen molecule, the magnitude of the newly developed attractive forces contributes more than the newly developed repulsive forces. As a result, the potential energy of the system begins to decrease.
- As the atoms come closer to one another the energy of the system decreases. The overlap of the atomic orbitals increases only up to a certain distance between the two nuclei, where the attractive and repulsive forces balance each other and the system attains minimum energy. At this stage, a stable bond is formed between the two atoms.
- If the distance between the two atoms is decreased further, the repulsive forces exceed the attractive forces and the energy of the system increases and stability decreases.
- When two hydrogen atoms with electrons having parallel spin approach each other, the potential energy of the system increases and bond formation does not take place.
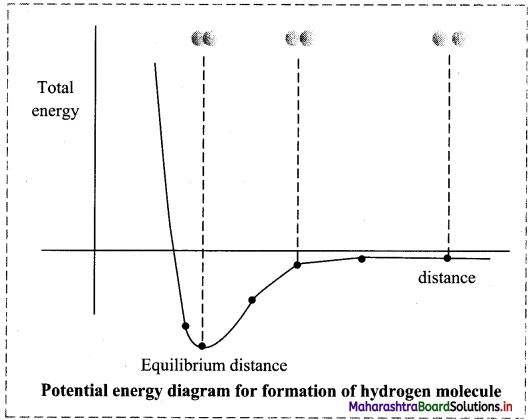
Question 32.
Define:
i. Sigma overlap
ii. pi overlap
Answer:
i. Sigma overlap (σ bond): When two half-filled orbitals of two atoms overlap along the internuclear axis, it is called as sigma overlap or sigma bond.
ii. pi overlap (π bond): When two half-filled orbitals of two atoms overlap side-ways (laterally), it is called as π overlap or π bond.
Question 33.
Explain with example:
i. s-s σ overlap
ii. p-p σ overlap
iii. s-p a overlap
Answer:
i. s-s σ overlap:
a. The overlap between two half-filled s orbitals of two different atoms containing unpaired electrons with opposite spins is called s-s overlap.
e.g. Formation of H2 molecule by s-s overlap:
Hydrogen atom (Z = 1) has electronic configuration: 1s1. The 1s1 orbitals of two hydrogen atoms overlap along the internuclear axis to form a σ bond between the atoms in H2 molecule.
b. Diagram:
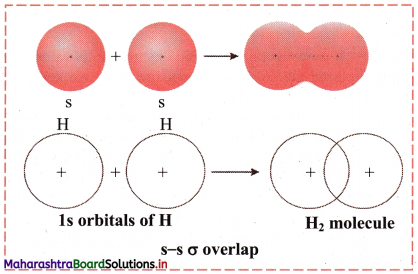
ii. p-p σ overlap:
a. This type of overlap takes place when two p orbitals from different atoms overlap along the internuclear axis.
e.g. Formation of F2 molecule by p-p overlap:
Fluorine atom (Z = 9) has electronic configuration 1s2 2s2 \(2 \mathrm{p}_{\mathrm{x}}^{2}\) \(2 \mathrm{p}_{\mathrm{y}}^{2}\) \(2 \mathrm{p}_{\mathrm{z}}^{1}\).
During the formation of F2 molecule, half-filled 2pz orbital of one F atom overlaps with similar half-filled 2pz orbital containing electron with opposite spin of another F atom axially and a p-p σ bond is formed.
b. Diagram:
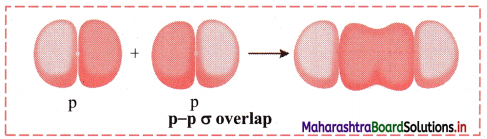
iii. s-p σ overlap:
a. In this type of overlap one half filled s orbital of one atom and one half filled p orbital of another orbital overlap along the internuclear axis.
e.g. Formation of HF molecule by s-p overlap:
Hydrogen atom (Z = 1) has electronic configuration: 1s1 and fluorine atom (Z = 9) has electronic configuration 1s2 2s2 \(2 \mathrm{p}_{\mathrm{x}}^{2}\) \(2 \mathrm{p}_{\mathrm{y}}^{2}\) \(2 \mathrm{p}_{\mathrm{z}}^{1}\). During the formation of HF molecule, half-filled 1s orbital of hydrogen atom overlaps coaxially with half-filled 2pz orbital of fluorine atom with opposite electron spin and an s-p σ bond is formed.
b. Diagram:
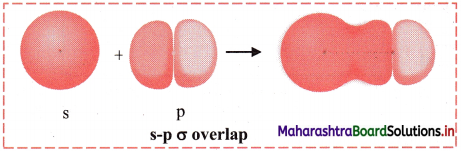
Question 34.
Explain the formation of π bond with diagram.
OR
Explain π overlap with diagram.
Answer:
- When two half-filled p orbitals of two atoms overlap side-ways (laterally), it is called π overlap and the bond formed is called π bond.
- π bond is perpendicular to the intemuclear axis.
Diagram:
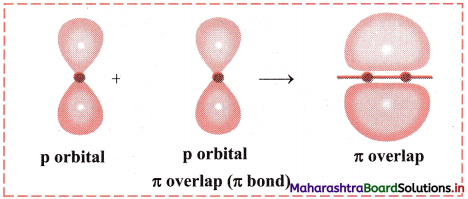
Question 35.
Identify the type of bond formed:
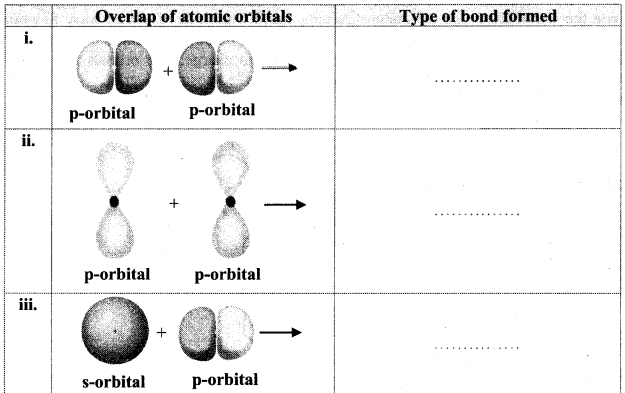
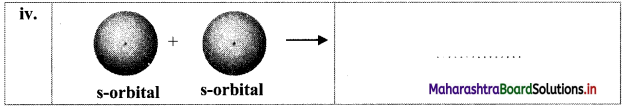
Answer:
i. σ bond
ii. π bond
iii. σ bond
iv. σ bond
![]()
Question 36.
Explain divalency of beryllium, though number of unpaired electrons in a beryllium atom is zero.
Answer:
Beryllium (Z = 4) has electronic configuration 1s2 2s2 in its ground state.
When one of the 2s electrons of Be is promoted to the vacant 2p orbital, the electronic configuration of Be in its excited state becomes 1s2 2s1 \(2 \mathrm{p}_{\mathrm{x}}^{1}\). This is called formation of an excited state and it has two unpaired electrons. Hence, though the number of unpaired electrons in the ground state of Be atom is zero, beryllium shows divalency.
Question 37.
Define the term hybridization.
Answer:
Hybridization is defined as the process of mixing of valence orbitals of same atom and recasting them into equal number of new equivalent orbitals (hybrid orbitals).
Question 38.
Explain in detail the steps involved in hybridization.
Answer:
Steps involved in hybridization:
i. Formation of the excited state:
a. The paired electrons in the ground state are uncoupled and one electron is promoted to the vacant orbital having slightly higher energy.
b. Now, total number of half-filled orbitals is equal to the valency of the element in the stable compound, e.g. In BeF2, valency of Be is two. In the excited state, one electron from 2s orbital is uncoupled and promoted to 2p orbital.

ii. Mixing and recasting:
- In this step, the two ‘s’ and ‘p’ orbitals having slightly different energies mix with each other.
- Redistribution of electron density and energy takes place and two new orbitals having exactly same shape and energy are formed.
- These new orbitals arrange themselves in space in such a way that there is minimum repulsion and maximum separation between them. e.g. During formation of sp hybrid orbitals as in Be, the two sp hybrid orbitals form an angle of 180° with each other.
Question 39.
List the important conditions required for hybridization.
Answer:
Conditions for hybridization:
- Orbitals belonging to the same atom can participate in hybridization.
- Orbitals having nearly same energy can undergo hybridization.
[Note: 2s and 2p orbitals of the same atom undergo hybridization but 3s and 2p orbitals of the same atom do not.]
Question 40.
Enlist the characteristic features of hybrid orbitals.
Answer:
Characteristic features of hybrid orbitals:
- Number of hybrid orbitals formed is exactly the same as the participating atomic orbitals.
- They have same energy and shape.
- Hybrid orbitals are oriented in space in such a way that there is minimum repulsion and thus are directional in nature.
- The hybrid orbitals are different in shape from the participating atomic orbitals, but they bear the characteristics of the atomic orbitals from which they are derived.
- Each hybrid orbitals can hold two electrons with opposite spins.
- A hybrid orbital has two lobes on the two sides of the nucleus. One lobe is large and the other small.
- Covalent bonds formed by hybrid orbitals are stronger than those formed by pure orbitals, because the hybrid orbital has electron density concentrated on the side with a larger lobe and the other is small allowing greater overlap of the orbitals.
![]()
Question 41.
Explain with diagrams:
i. sp3 hybridization
ii. sp2 hybridization
iii. sp hybridization
Answer:
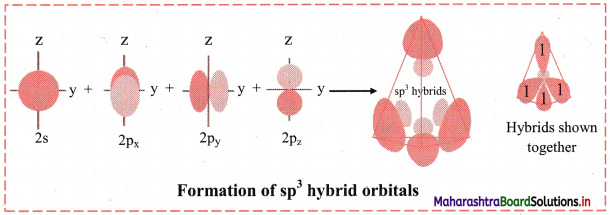
i. sp3 hybridization:
In this type, one s and three p orbitals having comparable energy mix and recast to form four sp3 hybrid orbitals, ‘s’ orbital is spherically symmetrical while the px, py, pz, orbitals have two lobes and are directed along x, y and z axes, respectively.
The four sp3 hybrid orbitals formed are equivalent in energy and shape. They have one large lobe and one small lobe. They are at an angle of 109° 28′ with each other in space and point towards the comers of a tetrahedron. CH4, NH3, H2O are examples where the orbitals on central atom undergo sp3 hybridization.
Diagram:
ii. sp2 hybridization:
This hybridization involves the mixing of one s and two p orbitals to give three sp2 hybrid orbitals of same energy and shape. The three orbitals are maximum apart and oriented at an angle of 120° and are in one plane. The third p orbital does not participate in hybridization and remains at right angles to the plane of the sp2 hybrid orbitals. BF3, C2H4 molecules are examples of sp2 hybridization.
Diagram:
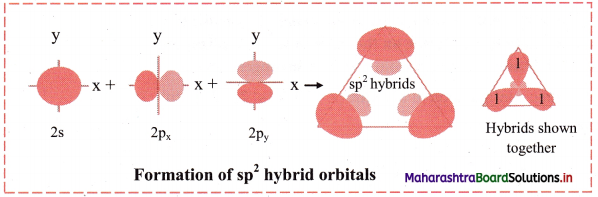
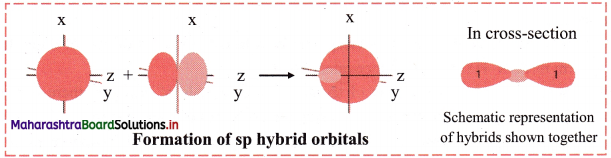
iii. sp hybridization:
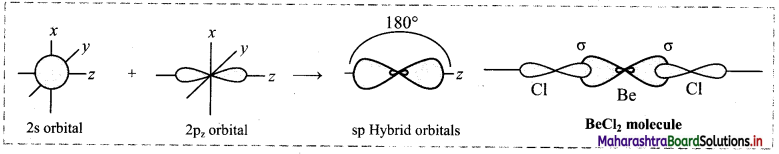
In this type, one s and one p orbital undergo mixing and recasting to form two sp hybrid orbitals of same energy and shape. The two hybrid orbitals are placed at an angle of 180°. Other two p orbitals do not participate in hybridization and are at right angles to the hybrid orbitals. For example, BeCl2 and acetylene molecule (HC ≡ CH).
Diagram:
Question 42.
Explain the formation of an ammonia molecule on the basis of hybridization.
Answer:
Formation of an ammonia (NH3) molecule on the basis of sp3 hybridization:
i. Ammonia molecule (NH3) has one nitrogen atom and three hydrogen atoms.
ii. The ground state electronic configuration of nitrogen (Z = 7) is 1s2 2s2 \(2 \mathrm{p}_{\mathrm{x}}^{1}\) \(2 \mathrm{p}_{\mathrm{y}}^{1}\) \(2 \mathrm{p}_{\mathrm{z}}^{1}\)
Electronic configuration of nitrogen:
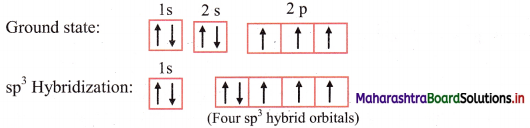
iii. The ground state electronic configuration explains the observed valency of nitrogen in NH3 which is three.
iv. The 2s, 2px, 2py and 2pz orbitals of nitrogen atom mix and recast to form four sp3 hybrid orbitals of equivalent energy. These orbitals are tetrahedrally oriented in space. One of the sp3 hybrid orbital contains a lone pair of electrons.
v. Three half-filled sp3 hybrid orbitals of N atom overlap axially with half-filled 1s orbital of three different hydrogen atoms to form three N-H (sp3-s) sigma covalent bonds.
vi. Since, there is one lone pair of electrons in one of the sp3 hybrid orbitals of nitrogen, there is repulsion between lone pair and bonding pair of electrons. As a result, the H-N-H bond angle is reduced from regular tetrahedral angle 109° 28′ to 107° 18′. Geometry of NH3 molecule is pyramidal or distorted tetrahedral.
Question 43.
Explain the formation of water (H2O) molecule on the basis of hybridization.
Answer:
Formation of water (H2O) molecule on the basis of sp3 hybridization:
i. Water molecule (H2O) has one oxygen atom and two hydrogen atoms.
ii. The ground state electronic configuration of oxygen (Z = 8) is 1s2 2s2 \(2 \mathrm{p}_{\mathrm{x}}^{2}\) \(2 \mathrm{p}_{\mathrm{y}}^{1}\) \(2 \mathrm{p}_{\mathrm{z}}^{1}\).
Electronic configuration of oxygen:
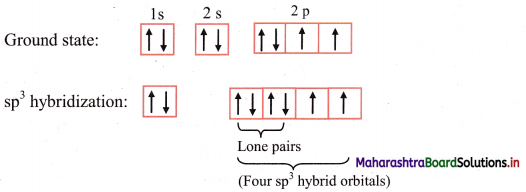
iii. The ground state electronic configuration explains the observed valency of oxygen in H2O molecule which is 2.
iv. The 2s, 2px, 2py and 2pz orbitals of oxygen atom mix and recast to form four sp3 hybrid orbitals of equivalent energy. These orbitals are tetrahedrally oriented in space. Two of the sp hybrid orbitals contain lone pair of electrons.
v. Two half-filled sp3 hybrid orbitals of O atom overlap axially with half-filled 1s orbitals of two different hydrogen atoms to fonn two O-H (sp3-s) sigma covalent bonds.
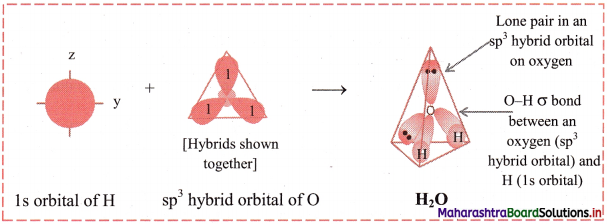
vi. Since, there are two lone pairs of electrons in two of the sp3 hybrid orbitals of oxygen, there is repulsion between lone pair and bonding pair of electrons. As a result, the H-O-H bond angle is reduced from regular tetrahedral angle 109°28′ to 104°35′. The geometry of H2O molecule is angular or V shaped.
Diagram:
Question 44.
Explain the formation of an ethene molecule on the basis of hybridization.
Answer:
Formation of an ethene (ethylene) molecule on the basis of sp2 hybridization:
i. Ethene molecule (C2H4) has two carbon atoms and four hydrogen atoms.
ii. The ground state electronic configuration of C (Z = 6) is 1s2 2s2 \(2 \mathrm{p}_{\mathrm{x}}^{1}\) \(2 \mathrm{p}_{\mathrm{y}}^{1}\) \(2 \mathrm{p}_{\mathrm{z}}^{0}\).
Electronic configuration of carbon:
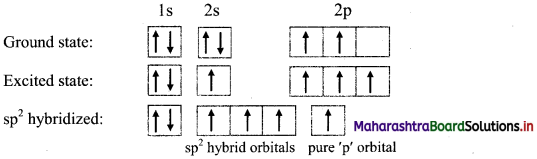
iii. One electron from 2s orbital of each carbon atom is excited to the 2pz orbital. Then each carbon atom undergoes sp2 hybridization.
iv. One ’s’ orbital and two ‘p’ orbitals on carbon hybridize to form three sp2 hybrid orbitals of equal energy and symmetry.
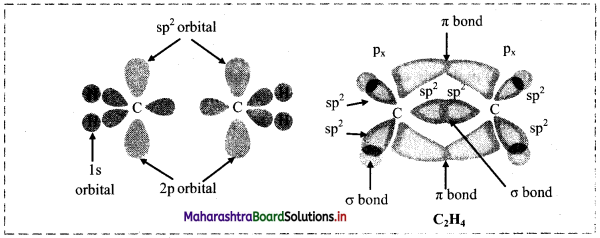
v. Two sp2 hybrid orbitals overlap axially two ‘s’ orbitals of hydrogen to form sp2-s σ bond. The unhybridized ‘p’ orbitals on the two carbon atoms overlap laterally to form a π bond. Thus, the C2H4 molecule has four sp2-s σ bonds, one sp2-sp2 σ bond and one p-p π bond.
vi. Each H-C-H and H-C-C bond angle in ethene molecule is 120°. All the six atoms in ethene (ethylene) molecule are in one plane. Geometry of the molecule at each carbon atom is trigonal planar.
![]()
Question 45.
Explain the formation of boron trifluoride on the basis of hybridization.
Answer:
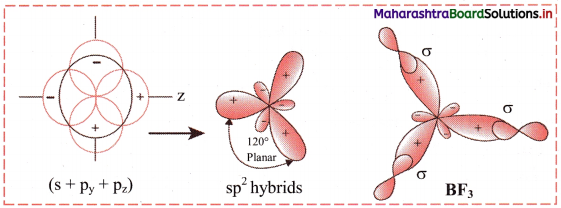
Formation of boron trifluoride on the basis of sp2 hybridization:
i. Boron trifluoride (BF3) has one boron atom and three fluorine atoms.
ii. Observed valency of boron in BF3 molecule is three and its geometry is trigonal planar. This can be explained on the basis of sp2 hybridization.
iii. The ground state electronic configuration of B (Z = 5) is 1s2 2s2 \(2 \mathrm{p}_{\mathrm{x}}^{1}\) \(2 \mathrm{p}_{\mathrm{y}}^{0}\) \(2 \mathrm{p}_{\mathrm{z}}^{0}\).
Electronic configuration of boron:
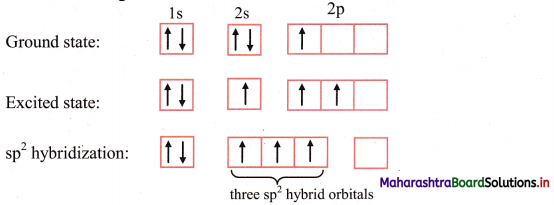
iv. One electron from 2s orbital of boron atom is uncoupled and promoted to vacant 2py orbital.
v. The three orbitals i.e. 2s, 2px of and 2py of boron undergoes sp2 hybridization to form three sp2 hybrid orbitals of equivalent energy, which are oriented along the three comers of an equilateral triangle making an angle of 120°.
vi. Each sp2 hybrid orbital of boron atom having unpaired electron overlaps axially with half-filled 2pz orbital of fluorine atom containing electron with opposite spin to form three B-F sigma bonds by sp2-p type of overlap.
vii. Each F-B-F bond angle in BF3 molecule is 120°. The geometry of BF3 molecule is trigonal planar.
Diagram:
Question 46.
Explain the formation of an acetylene molecule on the basis of hybridization.
Answer:
Formation of acetylene (ethyne) molecule on the basis of sp hybridization:
i. Acetylene molecule (C2H2) has two carbon atoms and two hydrogen atoms.
ii. The ground state electronic configuration of C (Z = 6) is 1s2 2s2 \(2 \mathrm{p}_{\mathrm{x}}^{1}\) \(2 \mathrm{p}_{\mathrm{y}}^{1}\) \(2 \mathrm{p}_{\mathrm{z}}^{0}\).
Electronic configuration of carbon:
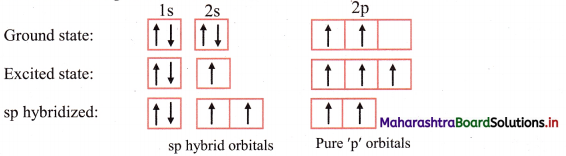
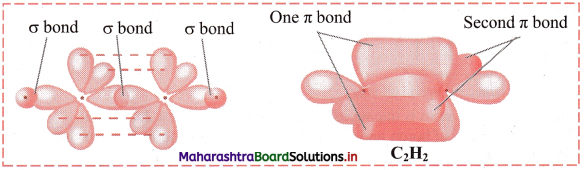
iii. Each carbon atom undergoes sp hybridization. One s and one p orbitals mix and recast to give two sp hybrid orbitals arranged at 180° to each other.
iv. Out of the two sp hybrid orbitals of carbon atom, one overlaps axially with s orbital of hydrogen while the other sp hybrid orbital overlaps with sp hybrid orbital of other carbon atom to form the sp-sp σ bond. The C H σ bond is formed by sp-s overlap.
v. The remaining two unhybridized p orbitals overlap laterally to form two p-p π bonds. So, there are three bonds between the two carbon atoms: one C-C σ bond (sp-sp) overlap, two C-C π bonds (p-p) overlap. There are two sp-s σ bonds in acetylene (one between each C and H).
vi. Each H-C-C bond angle in ethyne molecule is 180°. All the four atoms in ethyne molecule are in a straight line. The geometry of acetylene molecule is linear.
Diagram:
Question 47.
Explain the formation of BeCl2.
Answer:
Formation of BeCl2:
i. BeCl2 molecule has one Be atom and two chlorine atoms.
ii. Electronic configuration of Be is 1s2 2s2 \(2 \mathrm{p}_{\mathrm{z}}^{0}\).
Electronic configuration of beryllium:
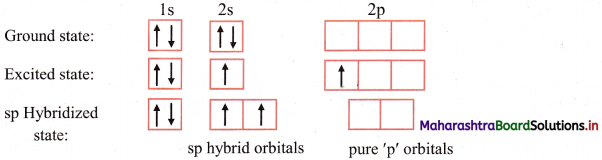
iii. The 2s and 2pz orbitals undergo sp hybridization to form two sp hybrid orbitals oriented at 180° with each other. 2pz orbitals of two chlorine atoms overlap with the sp hybrid orbitals to form two sp-p σ bonds.
Cl – Be – Cl bond angle is 180°. The geometry of the molecule is linear.
Diagram:
Question 48.
Match the following:
| Molecule | Hybridization and bond angle | ||
| i. | Water | a. | Sp2, 120° |
| ii. | Boron trifluoride | b. | Sp3, 104.5° |
| iii. | Beryllium fluoride | c. | Sp3, 109.5° |
| iv. | Methane | d. | Sp, 180° |
Answer:
i – b
ii – a
iii – d
iv – c
![]()
Question 49.
Give the importance of valence bond theory.
Answer:
Valence Bond theory introduced five new concepts in chemical bonding:
- Delocalization of electron over the two nuclei
- Shielding effect of electrons
- Covalent character of bond
- Partial ionic character of a covalent bond
- The concept of resonance and connection between resonance energy and molecular stability
Question 50.
What are the limitations of valence bond theory?
Answer:
Limitations of valence bond theory:
- Valence Bond theory explains only the formation of covalent bond in which a shared pair of electrons comes from two bonding atoms. However, it offers no explanation for the formation of a coordinate covalent bond in which both the electrons are contributed by one of the bonded atoms.
- Oxygen molecule is expected to be diamagnetic according to this theory. The two atoms in oxygen molecule should have completely filled electronic shells which give no unpaired electrons to the molecule making it diamagnetic. However, experimentally the molecule is found to be paramagnetic having two unpaired electrons. Thus, this theory fails to explain paramagnetism of oxygen molecule.
- Valence bond theory does not explain the bonding in electron deficient molecules like B2H6 in which the central atom possesses a smaller number of electrons than required for an octet of electron.
Question 51.
What are the two ways in which two atomic orbitals combine to form molecular orbitals (MOs)?
Answer:
Two atomic orbitals can combine in two ways to form molecular orbitals:
i. By addition of their wave functions.
ii. By subtraction of their wave functions.
Addition of the atomic orbtials wave functions results in formation of a molecular orbital which is lower in energy than atomic orbitals and is termed as Bonding Molecular Orbital (BMO). Subtraction of the atomic orbitals results in the formation of a molecular orbital which is higher in energy than the atomic orbitals and is termed as Antibonding Molecular Orbital (AMO).
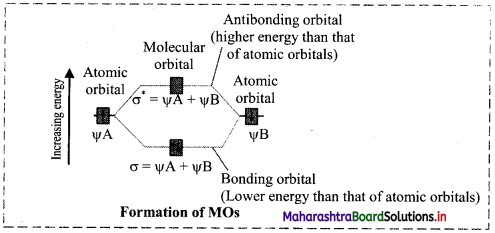
Question 52.
State True or False. Correct the false statement.
i. According to MO theory, the formation of molecular orbitals from atomic orbitals is expressed in terms of Linear Combination of Atomic Orbitals (LCAO).
ii. An MO contains maximum two electrons with opposite spins.
iii. Interference of electron waves of combining atoms can only be constructive.
iv. In bonding molecular orbital, the large electron density is observed between the nuclei of the bonding atoms than the individual atomic orbitals.
v. In the antibonding molecular orbital, the electron density is nearly zero between the nuclei.
Answer:
i. True
ii. True
iii. False
Interference of electron waves of combining atoms can be constructive or destructive.
iv. True
v. True
![]()
Question 53.
What are the conditions required for linear combination of atomic orbitals to form molecular orbitals?
Answer:
The following conditions are required for the linear combination of atomic orbitals (LCAO) to form molecular orbitals:
i. The combining atomic orbitals must have comparable energies.
So, Is orbitals of one atom can combine with 1 s orbital of another atom but not with 2s orbital, because energy of 2s orbital is much higher than that of 1 s orbital.
ii. The combining atomic orbitals must have the same symmetry along the molecular axis. Conventionally, z axis is taken as the internuclear axis. So even if atomic orbitals have same energy but their symmetry is not same they cannot combine. For example, 2s orbital of an atom can combine only with 2pz orbital of another atom, and not with 2px or 2py orbital of that atom because the symmetries are not same. pz is symmetrical along z axis while px is symmetrical along x axis.
iii. The combining atomic orbitals must overlap to the maximum extent. Greater the overlap, greater is the electron density between the nuclei and so stronger is the bond formed.
Question 54.
Explain and draw an energy level diagram obtained by the linear combination of two 1s atomic orbitals.
Answer:
The s-orbitals are spherically symmetrical along x, y and z axis. Two Is atomic orbitals combine to form σ 1s (bonding molecular orbital) and σ*1s (antibonding molecular orbital). Both the a bonding and σ* antibonding orbitals are symmetrical along the bond axis.
Diagram:
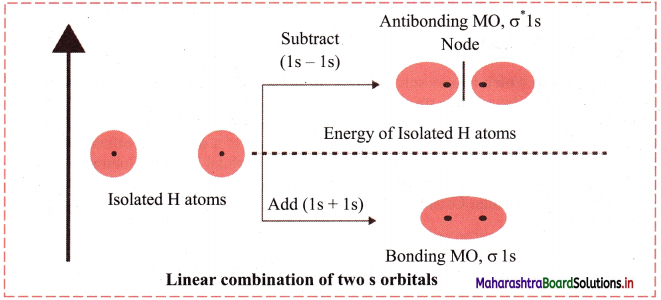
[Note: If we consider ‘z’ to be internuclear axis then linear combination of pz orbitals from two atoms can form σ 2pz bonding σ*(2pz) molecular orbitals.]
Question 55.
Explain the formation of π and π* molecular orbitals with the help of a diagram.
Answer:
When the atomic orbitals overlap laterally, a pi (π) molecular orbital is formed.
The px and py orbitals are not symmetrical along the bond axis. They have a positive lobe above the axis and negative lobe below the axis. Hence, linear combination of such orbitals leads to the formation of molecular orbitals with positive and negative lobes above and below the bond axis. These are designed as π bonding and π antibonding orbitals. The electron density in such π orbitals is concentrated above and below the bond axis. The π molecular orbitals has a node between the nuclei.
Diagram:
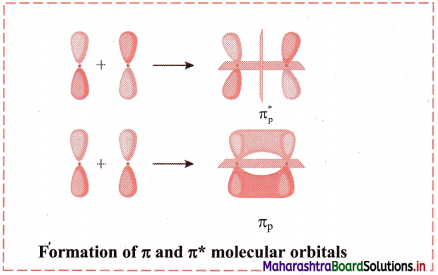
Question 56.
Write the increasing order of energies of molecular orbitals in various diatomic molecules of second row elements.
Answer:
The increasing order of energies of molecular orbitals for molecules (except O2 and F2) is:
σ1s < σ*1s < σ2s < σ*2s < (π2px = π2py) < σ2pz < (π*2px = π*2py) < σ*2pz
The increasing order of energy of molecular orbitals for diatomic molecules like O2 and F2 is:
σ1s < σ*1s < σ2s < σ*2s < σ2pz < (π2px = π2py) < (π*2px = π*2py) < σ*2pz
Question 57.
Explain briefly the information provided by the electronic configuration of molecules.
Answer:
The electronic configuration of molecules provides the following information:
- Stability of molecules: If the number of electrons in bonding MOs is greater than the number in antibonding MOs the molecule is stable.
- Magnetic nature of molecules: If all MOs in a molecule are completely filled with two electrons each, the molecule is diamagnetic (i.e., repelled) by magnetic field. However, if at least one MO is half-filled with one electron, the molecule is paramagnetic (i. e., attracted by magnetic field).
- Bond order of molecule: The bond order of the molecule can be calculated from the number of electrons in bonding MOs (Nb) and antibonding MOs (Na).
![]()
Question 58.
What are the key ideas of MO theory?
OR
What are the salient features of MO theory?
Answer:
Key ideas of MO Theory:
- MOs in molecules are similar to AOs of atoms. Molecular orbital describes region of space in the molecule representing the probability of an electron.
- MOs are formed by combining AOs of different atoms. The number of MOs formed is equal to the number of AOs combined.
- Atomic orbitals of comparable energies and proper symmetry combine to form molecular orbitals.
- MOs those are lower in energy than the starting AOs are bonding MOs and those higher in energy are antibonding MOs.
- The electrons are filled in MOs beginning with the lowest energy.
- Only two electrons occupy each molecular orbital and they have opposite spins, that is, their spins are paired.
- The bond order of the molecule can be calculated from the number of bonding and antibonding electrons.
Question 59.
Explain the formation of the following molecules on the basis of MOT. Also find the bond order.
i. H2
ii. Li2
iii. N2
iv. O2
v. F2
Answer:
i. Hydrogen molecule (H2):
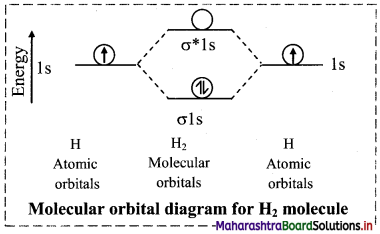
a. Hydrogen atom (Z = 1) has electronic configuration as 1s1.
b. Hydrogen atom contains one electron, hence hydrogen molecule which is diatomic contains two electrons.
c. Linear combination of two 1s atomic orbitals gives rise to two molecular orbitals σ1s and σ*1s.
d. The two electrons from the hydrogen atoms occupy the σ1s molecular orbital and σ*1s remains vacant.
e. Thus, electronic configuration of H2 molecule is σ1s2.
f. Since, no unpaired electron is present in hydrogen molecule, it is diamagnetic.
g. There are no electrons in the antibonding molecular orbital (σ*1s).
h. The bond order of H2 molecule is
Bond order = \(\frac{\mathrm{N}_{\mathrm{b}}-\mathrm{N}_{\mathrm{a}}}{2}=\frac{2-0}{2}\) = 1
Thus, a single covalent bond is present between two hydrogen atoms.
[Note: The bond length is 74 pm and the bond dissociation energy is 438 kJ mol-1.]
ii. Lithium molecule (Li2):
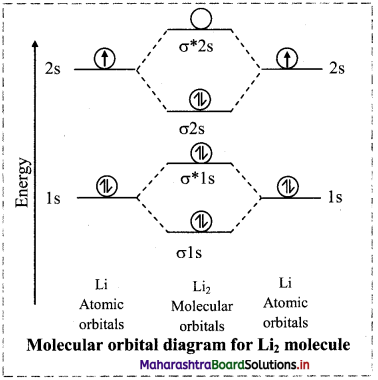
a. Lithium atom (Z = 3) has electronic configuration as 1s2 2s1.
b. Lithium atom has 3 electrons, hence Li2 molecule has 6 electrons.
c. Linear combination of four atomic orbitals gives rise to four molecular orbitals namely σ1s, σ*1s, σ2S and σ*2s.
d. The electronic configuration of Li2 molecule is (σ1s)2 (σ*1s)2 (σ2s)2.
e. Since no unpaired electron is present in lithium molecule, it is diamagnetic.
f. Bond order of Li2 molecule
\(=\frac{\mathrm{N}_{\mathrm{b}}-\mathrm{N}_{\mathrm{a}}}{2}=\frac{4-2}{2}=1\)
Thus, a single covalent bond is present between two Li atoms. Hence, Li2 is a stable molecule.
iii. Nitrogen molecule (N2):
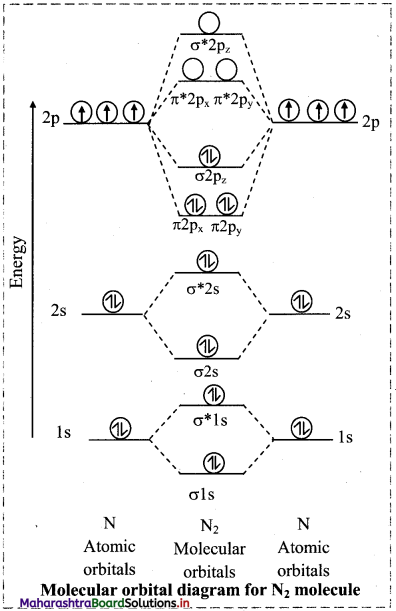
a. Nitrogen atom (Z = 7) has electronic configuration as 1s2 2s2 2p3.
b. Nitrogen atom contains 7 electrons, hence nitrogen molecule contains 14 electrons.
c. Linear combination of atomic orbitals gives rise to different molecular orbitals.
d. The electronic configuration of N2 molecule is
N2: (σ1s)2 (σ*1s)2 (σ2s)2 (σ*2s)2 (π2px)2 (π2py)2 (σ2pz)2
e. Since N2 molecule does not have unpaired electron, it is diamagnetic.
f. Bond order of N2 molecule
\(=\frac{\mathrm{N}_{\mathrm{b}}-\mathrm{N}_{\mathrm{a}}}{2}=\frac{10-4}{2}=3\)
Thus, there are three bonds in N2 molecule.
![]()
Question 60.
Study the following tables showing bond enthalpies of single and multiple bonds.
| Bond | ΔaH/kJ mol-1 |
| C-H | 400-415 |
| N-H | 390 |
| O-H | 460-464 |
| C-C | 345 |
| C-N | 290-315 |
| C-O | 355-380 |
| C-Cl | 330 |
| Bond | ΔaH/kJ mol-1 |
| C-Br | 275 |
| O-O | 175-184 |
| C=C | 610-630 |
| C≡C | 835 |
| C=O | 724-757 |
| C≡N | 854 |
i. Among single bonds, which bond is the strongest?
ii. How is bond enthalpy related to bond strength?
Answer:
i. Among single bonds, O-H bond is the strongest.
ii. Larger the bond enthalpy, stronger is the bond.
Question 61.
Write a short note on bond length.
Answer:
Bond length:
- Bond length is defined as the equilibrium distance between the nuclei of two covalently bonded atoms in a molecule.
- Each atom of the bonded pair contributes to the bond length.
- Bond length depends upon the size of atoms and multiplicity of bonds. It increases with increase in size of atom and decreases with increase in multiplicity of bond.
e.g. C – C single bond is longer than C ≡ C triple bond. - Bond lengths are measured by X-ray and electron diffraction techniques.
Question 62.
Cl-Cl covalent bond length is smaller than Br-Br covalent bond length. Explain.
Answer:
Bond length increases with increase in size of atom. Cl atom is smaller than Br atom. Hence, Cl-Cl covalent bond length is smaller than Br-Br covalent bond length.
Question 63.
Arrange the following bonds in decreasing order of bond strength: C-N, C=N, C≡N
Answer:
C≡N > C=N > C-N
Note: Average bond lengths for some single, double and triple bonds:
| Type of bond |
Covalent bond length (pm) |
| O-H | 96 |
| C-H | 107 |
| N-O | 136 |
| C-O | 143 |
| C-N | 143 |
| C-C | 154 |
| C=C | 121 |
| N=O | 122 |
| C=C | 133 |
| C=N | 138 |
| C≡N | 116 |
| C≡C | 120 |
| Type of bond |
Covalent bond length (pm) |
| H2(H-H) | 74 |
| F2(F-F) | 144 |
| Cl2(Cl-Cl) | 199 |
| Br2(Br-Br) | 228 |
| I2(I-I) | 267 |
| N2(N≡N) | 109 |
| O2(O-O) | 121 |
| HF (H-F) | 92 |
| HCl (H-Cl) | 127 |
| HBr (H-Br) | 141 |
| HI (H-I) | 160 |
Question 64.
Write a short note on bond order.
Answer:
i. According to the Lewis theory, bond order is given by the number of bonds between the two atoms in a molecule.
e.g. a. In hydrogen molecule, bond order between hydrogen atoms is one as one electron pair is shared.
b. In oxygen molecule, bond order between oxygen atoms is two as two electron pairs are shared.
c. In acetylene molecule, bond order between two carbon atoms is three as three electron pairs are shared.
ii. The isoelectronic molecules and ions have identical bond orders.
e.g. a. The bond order of F2 and \(\mathrm{O}_{2}{ }^{2-}\) is one.
b. The bond order of N2, CO and NO+ is 3.
iii. As the bond order increases, the bond enthalpy increases and bond length decreases.
iv. With the help of bond order, the stability of a molecule can be predicted.
[Note: N2 molecule has bond enthalpy of 946 kJ mol-1. It is one of the highest for diatomic molecules.]
![]()
Question 65.
Explain how polarity (ionic character) is developed in a covalent bond.
Answer:
i. Covalent bonds are formed between two atoms of the same or different elements.
ii. When a covalent bond is formed between atoms of same element such as H-H, F-F, Cl-Cl, etc., the shared pair of electrons is attracted equally to both atoms and is situated midway between two atoms. Such covalent bond is termed as nonpolar covalent bond.
iii. When a covalent bond is formed between two atoms of different elements that have different electronegativities, the shared electron pair does not remain at the centre. The electron pair is pulled towards the more electronegative atom resulting in the separation of charges. This give rise to dipole. The more electronegative atom acquires a partial -ve charge and the other atom gets a partial +ve charge. Such a bond is called as polar covalent bond. The examples of polar molecules include HF, HC1, etc.
![]()
Fluorine is more electronegative than hydrogen, therefore, the shared electron pair is more attracted towards fluorine and the atoms acquire partial +ve and -ve charges, respectively.
iv. Polarity of the covalent bond increases as the difference in the electronegativity between the bonded atoms increases. When the difference in electronegativities of combining atom is about 1.7, ionic percentage in the covalent bond is 50%.
Question 66.
Define and explain the term dipole moment.
Answer:
i. Dipole moment (μ) is the product of the magnitude of charge and distance between the centres of +ve and -ve charges.
ii. It is given by, µ = Q × r
where, Q = charge, r = distance of separation.
iii. Unit of dipole moment is Debye (D).
iv. Dipole moment being a vector quantity is represented by a small arrow with the tail on the positive centre and head pointing towards the negative centre.
![]()
Note: 1 D = 3.33564 × 10-30 C m
where C is coulomb and m is meter.
Question 67.
Dipole moment in case of BeF2 is zero. Explain.
Answer:
- Dipole moment is a vector quantity. Therefore, the resultant dipole moment of a molecule is the vector sum of dipole moments of various bonds in the molecule.
- In BeF2 molecule, Be-F bond is polar and has a bond dipole moment.
- BeF2 is a linear molecule with two Be-F bonds oriented at 180° (opposite to each other).
- The two bond dipoles are equal in magnitude and act in opposite direction to cancel each other. Therefore, the net dipole moment in case of BeF2 is zero.

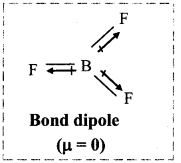
Question 68.
Dipole moment in case of BF3 is zero. Explain.
Answer:
i. Dipole moment is a vector quantity. Therefore, the resultant dipole moment of a molecule is the vector sum of dipole moments of various bonds in the molecule.
ii. In BF3 molecule, B-F bond is polar and has a bond dipole moment.
iii. Also, in BF3, the three B-F bonds are oriented at an angle of 120° to one another.
iv. The resultant of any two bond moments is equal in magnitude and opposite in direction to that of third. Hence, the net sum is zero and the dipole moment of tetra-atomic BF3 molecule is zero.
Question 69.
Dipole moment of H2O is higher than that of NH3. Explain.
Answer:
In both NH3 and H2O, the central atom undergoes sp3 hybridization. In both the molecules, the orbital dipole due to the lone pair increases the effect of resultant dipole moment. However, in NH3, nitrogen has only one lone pair while in H2O, oxygen has two lone pairs. Hence, dipole moment of H2O is higher than that of NH3.
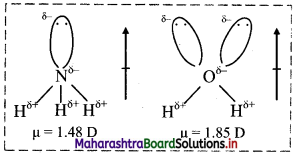
Question 70.
Dipole moment of NF3 is less than that of NH3, even though N-F bond is more polar than N-H bond. Explain.
Answer:
- Both NH3 and NF3 have pyramidal structure with a lone pair on the N atom. In NF3, F is more electronegative than N while in NH3, N is more electronegative.
- In NH3, the orbital dipole due to the lone pair is in the same direction as the resultant dipole moment of N-H bonds, whereas in NF3 the orbital dipole is in the direction opposite to the resultant dipole moment of three N-F bonds.
- The orbital dipole because of lone pair decreases the effect of the resultant N-F bond moments, which results in the low dipole moment of NF3 (0.8 × 10-30 C m) as compared to NH3 (4.90 × 10-30 C m).
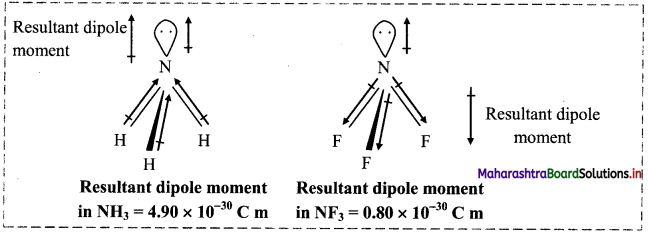
![]()
Question 71.
CHCl3 is polar. Explain.
Answer:
In CHCl3, the dipoles are not equal and do not cancel each other. Hence, CHCl3 is polar with a non-zero dipole moment.
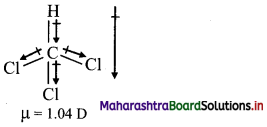
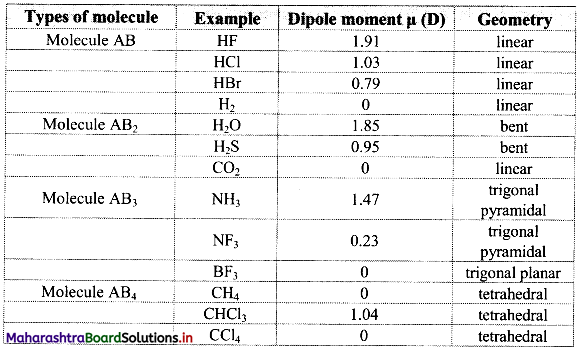
Note: Dipole moments and geometry of some molecules are given in the following table:
Question 72.
Explain Fajan’s rule with suitable examples.
Answer:
Fajan’s rule:
i. Smaller the size of the cation and larger the size of the anion, greater is the covalent character of the ionic bond. For example, Li+ Cl– is more covalent than Na+Cl. Similarly, Li+I– is more covalent than Li+Cl–.
ii. Greater the charge on cation, more is covalent character of the ionic bond. For example, covalent character of AlCl3, MgCl2 and NaCl decreases in the following order Al3+(Cl–)3 > Mg2+(Cl–)2 > Na+ Cl–
iii. A cation with the outer electronic configuration of the s2p6d10 type possesses greater polarising power compared to the cation having the same size and same charge but having outer electronic configuration of s2p6 type.
This is because d electrons of the s2p6d10 shell screen nuclear charge less effectively compared to s and p electrons of s2p6 shell. Hence, the effective nuclear charge in a cation having s2p6d10 configuration is greater than that of the one having s2p6 configuration. For example: Cu+Cl– is more covalent than Na+Cl–. Here,
(Cu+ = 1s2 2s2 2p6 3s2 3p6 3d10; Na+ = 1s2 2s2 2p6)
Question 73.
Explain resonance with respect to \(\mathrm{CO}_{3}^{2-}\) ion.
Answer:
i. Three structures written for \(\mathrm{CO}_{3}^{2-}\) as follows:
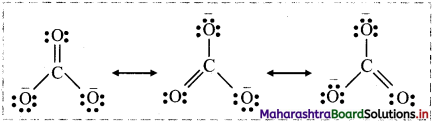
ii. Each structure differs from the other only in the position of electrons without changing positions of the atoms. None of these individual structures is adequate to explain the properties of \(\mathrm{CO}_{3}^{2-}\).
iii. The actual structure of \(\mathrm{CO}_{3}^{2-}\) is a combination of three Lewis structures and is called as the resonance hybrid.
iv. Energy of the resonance hybrid structure is less than the energy of any single canonical form. Hence, resonance stabilizes certain polyatomic molecules or ions.
v. The average of all resonating structures contributes to overall bonding characteristic features of the molecule or ion.
Question 74.
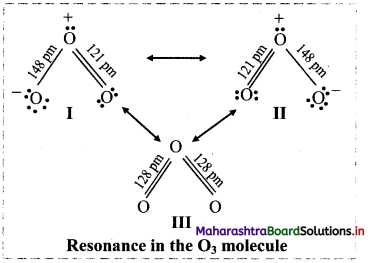
Explain O3 molecule is the resonance hybrid.
Answer:
Ozone is a resonance hybrid of structures I and II. The structures I and II are canonical forms while structure III is a resonance hybrid. The energy of structure III is less than that of I and II.
Question 75.
Define resonance energy.
Answer:
Resonance energy is defined as the difference in energy of the most stable contributing structure and the resonating forms.
![]()
Question 76.
Write the resonance structures of \(\mathrm{NO}_{3}^{-}\) ion.
Answer:
Resonance structures of \(\mathrm{NO}_{3}^{-}\) :
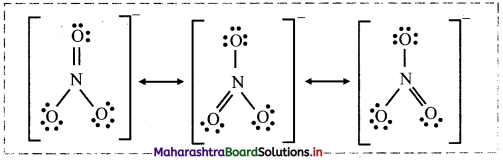
Question 77.
A student represents the Lewis dot structure of AlCl3 molecule as shown below:

i. Is the representation correct? Justify your answer.
ii. If the chlorine atoms are replaced by bromine atoms, what will be the number of electrons present in the valence shell of aluminium?
Answer:
i. No, the representation is incorrect. There will be no lone pair of electrons on aluminium.
ii. The number of electrons present in the valence shell of aluminium will be six.
Question 78.
Below is an incomplete Lewis structure for glycine. Complete the following Lewis structure and answer the following questions. (Hint: Add lone pairs and multiple bonds to the structure below to give each atom a formal charge of zero.)
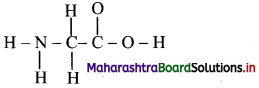
i. How many lone pairs of electrons are present on N-atom in the structure?
ii. How many pi bonds are present in the structure?
iii. How many sigma bonds are present in the structure?
Answer:
The correct Lewis structure is:
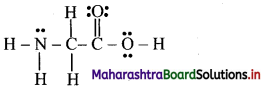
i. The number of lone pairs of electrons on N-atom is 1.
ii. The number of pi bonds in the structure is 1.
iii. The number of sigma bonds in the structure is 9.
Question 79.
Consider the following four species and answer the below given questions.
\(\mathrm{O}_{2}^{-}\), O2 \(\mathrm{O}_{2}^{+}\), \(\mathrm{O}_{2}^{2-}\)
i. What is the bond order of \(\mathrm{O}_{2}^{+}\) ?
ii. Which species is least stable?
Answer:
i. Electronic configuration of \(\mathrm{O}_{2}^{+}\) can be given as:
(σ1s)2 (σ*1s)2 (σ2s)2 (σ*2s)2 (σ2pz)2 (π2px)2 (π2py)2 (π*2px)1 (π*2py)0
∴ Bond order = \(\frac {1}{2}\) (10 – 5) = 2.5
ii. Stability of the molecule or species ∝ Bond order
Bond order decreases as: \(\mathrm{O}_{2}^{+}\) > O2 > \(\mathrm{O}_{2}^{-}\) > \(\mathrm{O}_{2}^{2-}\)
∴ Stability decreases as: \(\mathrm{O}_{2}^{+}\) > O2 > \(\mathrm{O}_{2}^{-}\) > \(\mathrm{O}_{2}^{2-}\)
Hence, least stable species is \(\mathrm{O}_{2}^{2-}\).
![]()
Multiple Choice Questions
1. The CORRECT Lewis structure of CO molecule is:
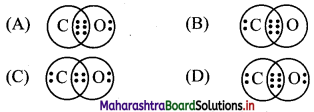
Answer:
2. Which of the following molecule does NOT obey octet rule?
(A) BF3
(B) CO2
(C) H2O
(D) N2
Answer:
(A) BF3
3. In BF3, bond angle is .
(A) 90°
(B) 109°
(C) 120°
(D) 180°
Answer:
(C) 120°
4. Identify the geometry represented by the following diagram.
(A) Trigonal bipyramidal
(B) T-shape
(C) square planar
(D) square pyramidal
Answer:
(D) square pyramidal
![]()
5. The geometry of H2S is ………….
(A) tetrahedral
(B) angular
(C) linear
(D) trigonal planar
Answer:
(B) angular
6. What will be the shape of molecule whose central atom is associated with 3 bonds and one lone pair?
(A) Trigonal pyramidal
(B) Tetrahedral
(C) Square planar
(D) Triangular planar
Answer:
(A) Trigonal pyramidal
7. Pair of molecules having identical geometry is …………..
(A) BF3, NH3
(B) BF3, AlF3
(C) BeF2, H2O
(D) BCl3, PCl3
Answer:
(B) BF3, AlF3
8. Which of the following molecule has bent shape?
(A) PCl3
(B) OF2
(C) BH3
(D) BeBr2
Answer:
(B) OF2
9. Which of the following is INCORRECT?
(A) The strength of the bond depends on the extent of overlap of the atomic orbitals.
(B) The extent of overlap depends on the shape and size of the atomic orbitals.
(C) The energy of the bonded atoms is more than that of the free atoms.
(D) During overlap of atomic orbitals, the electron density increases in between the two nuclei.
Answer:
(C) The energy of the bonded atoms is more than that of the free atoms.
![]()
10. In the potential energy curve for hydrogen molecule, the maximum stability is achieved when …………..
(A) potential energy of the system is maximum
(B) potential energy of the system is minimum
(C) force of repulsion become greater than force of attraction
(D) no bond formation takes place
Answer:
(B) potential energy of the system is minimum
11. In acetylene, C-C σ bond is formed by …………. overlap.
(A) sp2-sp2
(B) sp-sp
(C) sp-s
(D) p-p
Answer:
(B) sp-sp
12. The formation of O-H bonds in a water molecule involves …………. overlap.
(A) sp3-s
(B) sp1-s
(C) sp-p
(D) sp3-p
Answer:
(A) sp3-s
13. The molecular orbital shown in the diagram can be described as ………….
(A) σ
(B) σ*
(C) π*
(D) π
Answer:
(C) π*
14. The bond order of lithium molecule is ………….
(A) one
(B) two
(C) three
(D) four
Answer:
(A) one
15. The bond order in N2 molecule is …………
(A) 1
(B) 2
(C) 3
(D) 4
Answer:
(C) 3
![]()
16. The bond energies of F2, Cl2, Br2 and I2 are 37, 58, 46, and 36 kcal/mol respectively. The strongest bond is present in …………..
(A) Br2
(B) I2
(C) Cl2
(D) F2
Answer:
(C) Cl2
17. The common features among the species CO and NO+ are: ……………
(A) isoelectronic species and bond order 3
(B) isoelectronic species and bond order 2
(C) odd electron species and unstable
(D) odd electron species and bond order 1
Answer:
(A) isoelectronic species and bond order 3
18. Which of the following is CORRECT for H2O ?
| H=O bond | H2O molecule | |
| (A) | polar | nonpolar |
| (B) | nonpolar | polar |
| (C) | polar | polar |
| (D) | nonpolar | nonpolar |
Answer:
(C)
19. Each of the following molecules has a non-zero dipole moment EXCEPT:
(A) NF3
(B) BF3
(C) SO2
(D) LiH
Answer:
(B) BF3
20. Which of the following compounds is non-polar?
(A) HCl
(B) CH2Cl2
(C) CHCl3
(D) CCl4
Answer:
(D) CCl4
![]()
21. The dipole moment of …………..
(A) NF3 is higher than that of NH2
(B) BF3 is higher than that of NH3
(C) H2S is higher than that of H2O
(D) HCl is higher than that of HBr
Answer:
(D) HCl is higher than that of HBr