Balbharti Maharashtra State Board 11th Biology Important Questions Chapter 6 Biomolecules Important Questions and Answers.
Maharashtra State Board 11th Biology Important Questions Chapter 6 Biomolecules
Question 1.
How are living organisms classified? Give examples of each.
Answer:
1. Living organisms are classified as unicellular (consisting of single-cell) and multicellular (having many cells).
2. Example of unicellular organisms: bacteria, yeast.
Examples of multicellular organisms: plants, animals.
![]()
Question 2.
What is biochemistry?
Answer:
1. Biochemistry is biological chemistry that provides us the idea of the chemistry of living organisms and molecular basis for changes taking place in plants, animals and microbial cells.
2. It develops the foundation for understanding all biological processes and communication within and between cells as well as chemical basis of inheritance and diseases in animals and plants.
Question 3.
What does chemical analysis of living organisms indicate?
Answer:
Chemical analysis of all living organisms indicates the presence of the most common elements like carbon, hydrogen, nitrogen, oxygen, sulphur, calcium, phosphorus, magnesium and others with their respective content per unit mass of a living tissue.
Question 4.
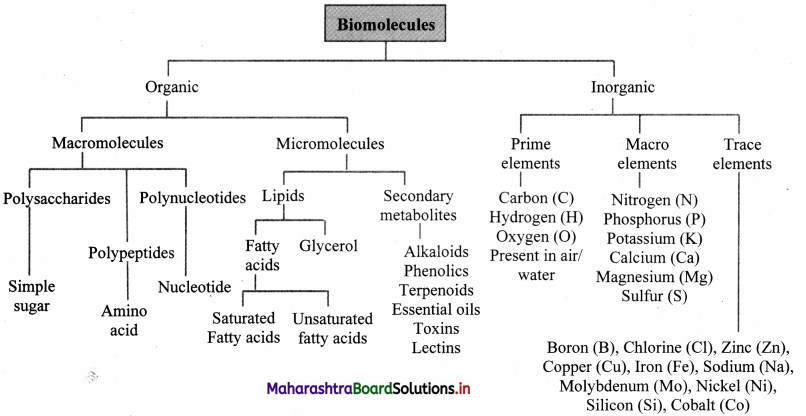
Name the basic macromolecules present in the living organisms.
Answer:
Polysaccharides (carbohydrate) polymer of monosaccharide, polypeptides (proteins) polymer of amino acids and polynucleotides (nucleic acids) polymer of nucleotides are the three basic macromolecule present in the living organisms.
Question 5.
Draw a flowchart showing classification of carbohydrates.
Answer:
![]()
Question 6.
Write a short note on
1. Glucose
2. Galactose and
3. Fructose.
Answer:
1. Glucose:
a. It is the most important fuel in living cells.
b. Its concentration in the human blood is about 90mg per 100ml of blood.
c. The small size and solubility in water of glucose molecules allows them to pass through the cell membrane into the cell.
d. Energy is released when the molecules are metabolized by cellular respiration.
2. Galactose:
a. It looks very similar to glucose molecules.
b. They can also exist in a and p forms.
c. Galactose react with glucose to form the disaccharide lactose.
d. However, glucose and galactose cannot be easily converted into one another.
e. Galactose cannot play the same role in respiration as glucose.
3. Fructose:
a. It is the fruit sugar and chemically it is ketohexose but it has a five-atom ring rather than a six-atom ring.
b. Fructose reacts with glucose to form the sucrose, a disaccharide.
Question 7.
How are disaccharides absorbed through the cell membrane?
Answer:
1. Disaccharides are soluble in water but they are too big to pass through the cell membrane by diffusion.
2. They are broken down in the small intestine during digestion.
3. Thus, formed monosaccharides then pas into the blood and through cell membranes into the cells.
![]()
Question 8.
Identify the X and Y in the following structure of a disaccharide.
Answer:
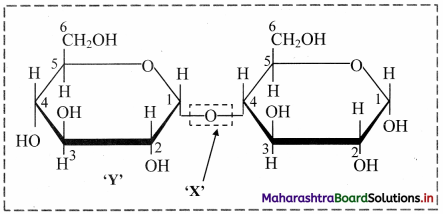
X: Glycosidic bond Y: Glucose
Question 9.
Distinguish between monosaccharides and disaccharides.
Answer:
| Monosaccharides | Disaccharides |
| 1. They are composed of 3-6 carbon atoms. | They are composed of two monosaccharide units covalently linked to each other. |
| 2. They cannot be hydrolyzed into smaller units. | They can be hydrolysed into monosaccharides. |
| 3. Glucose, Fructose | Sucrose and Lactose |
Question 10.
Which macromolecules are too big to escape from the cell?
Answer:
Polysaccharides are too big to escape from the cell.
![]()
Question 11.
Write a short note on
1. Starch
2. Glycogen
3. Cellulose.
Answer:
1. Starch:
a. Starch is a stored food in the plants.
b. Starch contains two types of glucose polymer: amylose and amylopectin.
c. Both are made from a-glucose.
d. Amylose is an unbranched polymer of a-glucose.
e. The molecules coil into a helical structure.
f. It foims a colloidal suspension in hot water.
g. Amylopectin is a branched polymer of a-glucose.
h. It is completely insoluble in water.
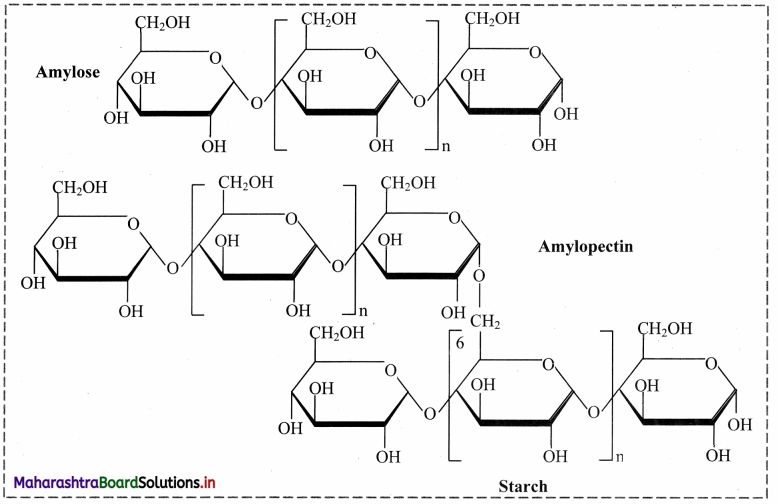
2. Glycogen:
a. It is amylopectin with very short distances between the branching side-chains.
b. Glycogen is stored in animal body particularly in liver and muscles from where it is hydrolyzed as per need to produce glucose.
3. Cellulose:
a. It is a polymer made from P-glucosc molecules and the polymer molecules are ‘straight’.
b. Cellulose serves to form the cell walls in plant cells.
c. These are much tougher than cell membranes.
d. This toughness is due to the arrangement of glucose units in the polymer chain and the hydrogen-bonding between neighbouring chains.
![]()
Question 12.
Why plant fats are liquid at room temperature while animal fats are solid?
Answer:
- Plant fats are unsaturated fatty acids, whereas animal fats are saturated fatty acids.
- Fats having unsaturated fatty acids are liquid at room temperature.
- Saturated fatty acids are solid at room temperature. Hence, plant fats are liquid at room temperature, while animal fats are solid.
Question 13.
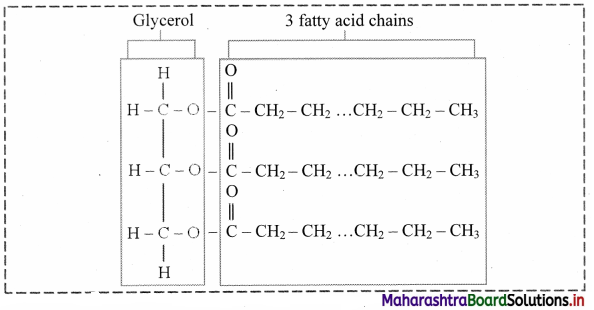
Draw the structure of triglyceride.
Answer:
Question 14.
Observe the following diagram and answer the questions based on it.
1. Identify the part ‘X’ in the given diagram.
2. What is the chemical property of the part ‘X’.
Answer:
1. The part labelled as ‘X’ is non-polar tail.
2. Non-polar tails are hydrophobic in nature.
Question 15.
Give two examples of unsaturated fatty acids.
Answer:
Oleic acid and linoleic acid are the examples of unsaturated fatty acids.
![]()
Question 16.
Explain primary structure of protein.
Answer:
The linear sequence of amino acids in polypeptide chain of a protein forms the primary structure of a protein.
Question 17.
Explain the secondary structure of protein with examples.
Answer:
- There are two types of secondary structure of protein: a-helix and P-pleated sheets.
- The polypeptide chain is arranged in a spiral helix. These spiral helices are of two types: a-helix (right handed) and P-helix (left handed).
- This spiral configuration is held together by hydrogen bonds.
- The sequence of amino acids in the polypeptide chain determines the location of its bend or fold and the position of formation of hydrogen bonds between different portions of the chain or between different chains. Thus, peptide chains form an a-helix structure.
- Example of a-helix structure is keratin.
- In some proteins two or more peptide chains are linked together by intermolecular hydrogen bonds. Such structures are called P-pleated sheets.
- Example of P-pleated sheet is silk fibres.
- Due to formation of hydrogen bonds peptide chains assume a secondary structure.
Question 18.
Explain the tertiary and quaternary structure of protein with example.
Answer:
Tertiary structure:
- In tertiary structure the peptide chains are much looped, twisted and folded back on themselves due to formation of disulphide bonds.
- Such loops and bends give the protein a tertiary structure.
- E.g. Myoglobin, enzymes.
Quaternary structure:
1. When a protein has more than two polypeptide subunits their arrangement in space is called quaternary structure.
2. E.g. Haemoglobin.
Question 19.
Write a note on properties of protein.
Answer:
Properties of proteins are as follows:
- Proteins are extremely reactive and highly specific in behaviour.
- Proteins are amphoteric in nature i.e. they act as both acids and bases.
- The behaviour of proteins is strongly influenced by pH.
- Like amino acids, proteins are dipolar ions at the isoelectric point i.e. the sum of the positive charges is equal to the sum of the negative charges and the net charge is zero.
- The ionic groups of a protein are contributed by the side chains of the polyfunctional amino acids.
- A protein consists of more basic amino acids such as lysine and arginine exist as a cation at the physiological pH of 7.4. Such proteins are called basic proteins.
- Histones of nucleoproteins are basic proteins.
- A protein rich in acidic amino acids exists as an anion at the physiological pH. Such proteins are called acidic proteins.
- Most of the blood proteins are acidic proteins.
Question 20.
Mention the findings of Feulgen.
Answer:
1. In 1924, Feulgen showed that chromosomes contain DNA.
2. He found that nucleic acids contain two pyrimidine (cytosine and thymine) and two purine (adenine and guanine) bases.
![]()
Question 21.
What were the findings of Wilkins and coworkers?
Answer:
The findings of Wilkins and coworkers were as follows:
- Purine and pyrimidine bases are placed regularly along the DNA molecules at a distance of 3.4 A.
- DNA (Deoxyribonucleotide) is composed of sugar molecule (a pentose sugar of deoxyribose type), phosphoric acid (phosphates when in chemical combination), nitrogen containing bases (nitrogen containing organic ring compounds).
- Bases are of two types: Pyrimidine bases and purine bases.
- Pyrimidine bases are single ring (monocyclic) nitrogenous bases. Cytosine, Thymine and uracil are pyrimidines.
- Purine are double ring (dicyclic) nitrogenous bases. Adenine and guanine are purines.
Question 22.
Chargaff analyzed the composition of DNA from various sources. Mention what were his implications from all his experiments.
Answer:
Implications proposed by Erwin Chargaff:
1. Purine and pyrimidine always occur in equal amount in DNA.
2. The base ratio i.e. A+T/G+C may vary in the DNA of different groups of animals and plants but the ratio remains constant for particular species.
Question 23.
Describe the structure of DNA.
Answer:
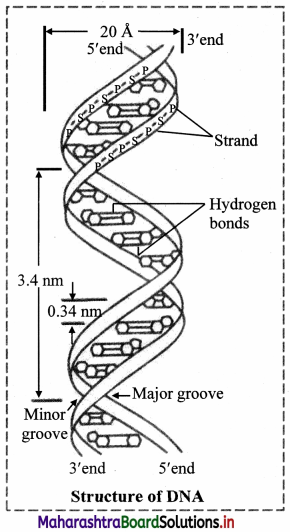
- DNA is a long chain made up of alternate sugar and phosphate groups. The sugar present in DNA is always a deoxyribose attached to a phosphate group. So, it forms a regular, repeating phosphate sugar sequence.
- A base is attached to sugar -phosphate chain. Together this unit which consist of sugar, phosphate and a base is called nucleotide.
- The nitrogenous base and a sugar of a nucleotide form a molecule called nucleoside. It lacks phosphate group. Four types of nucleoside are found in DNA molecule.
- In a nucleoside, nitrogenous base is attached to the first carbon atom (C-1) of the sugar and when a phosphate group gets attached with that of the carbon (C-5) atom of the sugar molecule a nucleotide molecule is formed.
- A single strand of DNA consists of several thousands of nucleotides one above the other.
- The phosphate group of the lower nucleotide attached with the 5th carbon atom of the deoxyribose sugar forms phospho-di-ester bond with that of the 3rd carbon atom of the deoxyribose sugar of the nucleotide placed just above it.
- Single long chain of polynucleotides of DNA consists of one end with sugar molecules not connected with another nucleotide having C-3 carbon which is not connected with phosphate group, similarly the other end having C-5 of the sugar is not connected with any phosphate group. These two ends of the polynucleotide chain are called as 3′ and 5′ ends respectively.
- The single polynucleotide strand of DNA is not straight but helical in shape.
- The DNA molecule consists of such two helical polynucleotide chains which are complementary to each other.
- The two complementary polynucleotide chains of DNA are held together by the weak hydrogen bonds.
- Adenine always pairs with thymine, and guanine with cytosine (a pyrimidine with a purine).
- Adenine-thymine pair consists of two hydrogen bonds and guanine-cytosine pair consists of three hydrogen bonds (Thus, if the sequence of bases of a polynucleotide chain is known, that of the other can be determined).
Question 24.
Draw the structures of nitrogen bases in nucleic acid.
Answer:
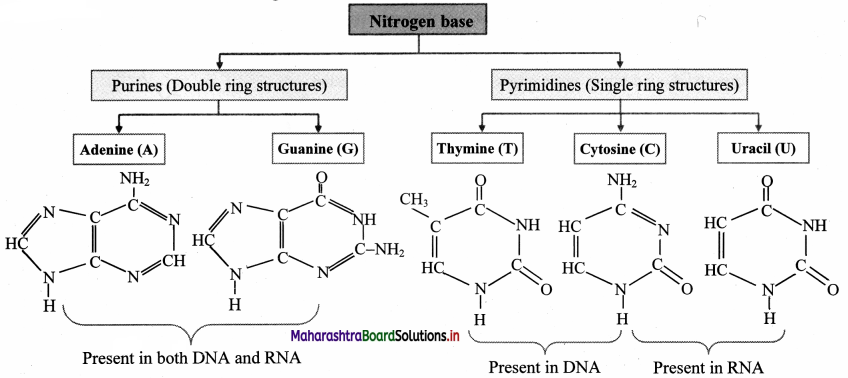
![]()
Question 25.
Describe the structure of RNA.
Answer:
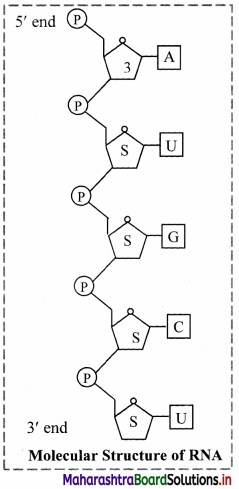
- The other nucleic acid found in living organisms is Ribose nucleic acid.
- In most of the organisms it is not found to be hereditary material but in certain organisms like tobacco mosaic virus, it is the hereditary material.
- Like DNA, ribose nucleic acid also consists of polynucleotide chain with the difference that it consists of single strand. Exceptions are Reovirus and wound tumor virus where RNA is double stranded.
- The nucleotides of RNA have ribose sugar instead of the deoxyribose sugar as in the case of DNA.
- In case of RNA, Uracil substitutes thymine of DNA.
- Purine, pyrimidine equality is not found in RNA molecule because of its single stranded structure.
- RNA strand is usually found folded upon itself in certain regions or entirely. These folding helps in stability of the RNA molecule.
- Most of the RNA polynucleotide chains start either with adenine or guanine.
- Three types of cellular RNAs have been distinguished:
- messenger RNA (mRNA) or template RNA,
- ribosomal RNA (rRNA),
- transfer RNA (tRNA) or soluble RNA.
Question 26.
Observe the following figure and name the type of bond shown by arrow in the structure.
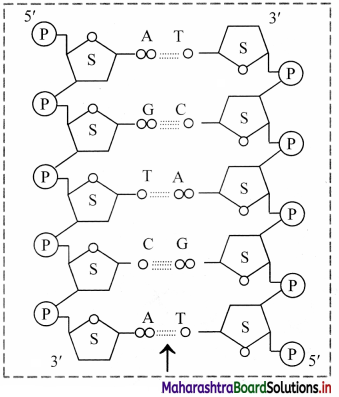
Answer:
The type of bond shown in the diagram is hydrogen bond.
![]()
Question 27.
What would have happened if there were no enzymes in the body?
Answer:
If enzymes were absent in the body, either the reactions would not occur or if they occur they would occur at a very slow rate.
Question 28.
How many reactions are catalyzed by an enzyme?
Answer:
Each enzyme catalyzes only one reaction.
Question 29.
What is a substrate?
Answer:
The substance upon which an enzyme acts is termed as the substrate.
Question 30.
What is endo-enzyines? Give examples.
Answer:
The enzymes which act within the cell in which they are synthesized are known as endo-enzymes E.g., enzymes produced in the chloroplast and mitochondria.
Question 31.
What are exo-enzymes?
Answer:
1. The enzymes which act outside the cell of which they are synthesized are known as exo-enzymes. E.g. enzymes released by many fungi.
2. These enzymes, synthesized by living cell, retain their catalytic property even when extracted from cells.
Question 32.
How are enzymes categorised?
Answer:
On basis of chemical composition enzymes are categorised:
1. Purely proteinaceous enzymes: e.g. Proteases that spilt protein
2. Conjugated enzymes: enzymes are made up of a protein to which a non-protein prosthetic group is attached.
![]()
Question 33.
What is a prosthetic group ? What w ill happen if it is removed?
Answer:
1. Prosthetic group is non-protein in nature and is attached to the protein component of enzyme by chemical bonds.
2. It is not removed by hydrolysis.
3. If the prosthetic group is removed the protein part of the enzyme becomes inactive.
Question 34.
What are coenzymes?
Answer:
1. Enzymes require certain organic compounds for their activity.
2. The organic compounds that are tightly attached to the protein part are called coenzymes.
3. E.g. Nicotinamaide adenine dinucleotide (NAD), Flavin mononucleotide (FMN).
Question 35.
What are co-factors?
Answer:
1. Enzymes require certain inorganic ions for their activity.
2. The inorganic ions which are loosely attached to the protein part are called co-factors.
E.g. Magnesium, copper, zinc, iron, manganese etc.
[Note: Apoenzyme (protein part) and co-factor together form a complete catalytically active enzyme which is known as holoenzyme. The co-factors are of two types; metal ions and coenzymes. A coenzyme or metal ion that is very tightly or even covalently bound to the enzyme protein is known as a prosthetic group.]
Question 36.
Complete the analogy.
Iron (Fe): Catalase: Manganese (Mn):
Answer:
Peptidase
Question 37.
Give examples of coenzymes and cofactors.
Answer:
1. Nicotinamaide adenine dinucleotide (NAD), Flavin mononucleotide (FMN).
2. Magnesium, copper, zinc, iron, manganese etc.
![]()
Question 38.
How are enzymes named?
Answer:
- Enzymes are named by adding the suffix- ‘ase’ to the name of the substrate on which they act e.g. protease, sucrase, nuclease etc. which break up proteins, sucrose and nucleic acids respectively.
- The enzymes can be named according to the type of function they perform.
For e.g., dehydrogenase remove hydrogen, carboxylase add CO; decarboxylases remove C02, oxidases helping in oxidation. - Some enzymes are named according to the source from which they are obtained.
For e.g., papain from papaya, bromelain from the member of Bromeliaceae family, pineapple. - According to international code of enzyme nomenclature, the name of each enzyme ends with an -ase and consists of double name!
- The first name indicates the nature of substrate upon which the enzyme acts and the second name indicates the reaction catalyzed.
For e.g., pyruvic decarboxylase catalyses the removal of C02 from the substrate pyruvic acid.
Similarly, the enzyme glutamate pyruvate transaminase catalyses the transfer of an amino group from the substrate glutamate to another substrate pyruvate.
Question 39.
Explain in detail the mechanism of enzyme action. Write a note on model proposed by Emil Fischer for mechanism of enzyme action.
Answer:
1. The basic mechanism by which enzymes catalyze chemical reactions begins with the binding of the substrate (or substrates) to the active site on the enzyme.
2. The active site is the specific region of the enzyme which combines with the substrate.
3. The binding of the substrate to the enzyme causes changes in the distribution of electrons in the chemical bonds of the substrate and ultimately causes the reactions that lead to the formation of products.
4. The products are released from the enzyme surface to regenerate the enzyme for another reaction cycle.
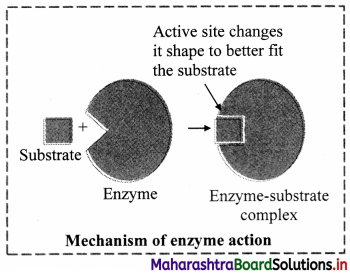
5. Lock and Key model proposed by Emil Fischer: i. Proteinaceous Nature:
All enzymes are basically made up of protein.
![]()
Question 40.
Describe the concept of metabolism.
Answer:
- Metabolism is the sum of the chemical reactions that take place within each cell of a living organism and provide energy for vital processes and for synthesizing new organic material.
- It involves continuous process of breakdown and synthesis of biomolecules through chemical reactions.
- Each of the metabolic reaction results in a transformation of biomolecules.
- Most of these metabolic reactions do not occur in isolation but are always linked with some other reactions.
- In cells, metabolism involves two following types of pathways:
a. Catabolic pathways
This involves formation of simpler structure from a complex biomolecule.
For e.g. when we eat wheat, bread or chapati, our gastrointestinal tract digests (hydrolyses) the starch to glucose units with help of enzymes and releases energy in form of ATP (Adenosine triphosphate).
b. Anabolic pathway
It is also called as biosynthetic pathway that involves formation of a more complex biomolecules from a simpler structure
Fone.g., synthesis of glycogen from glucose and protein from amino acids. These pathways consume energy.
Question 41.
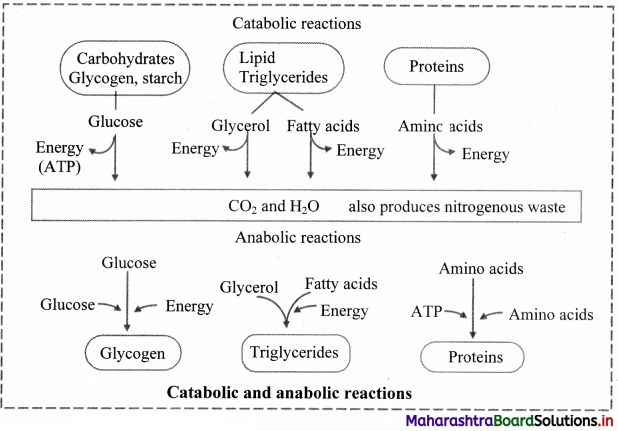
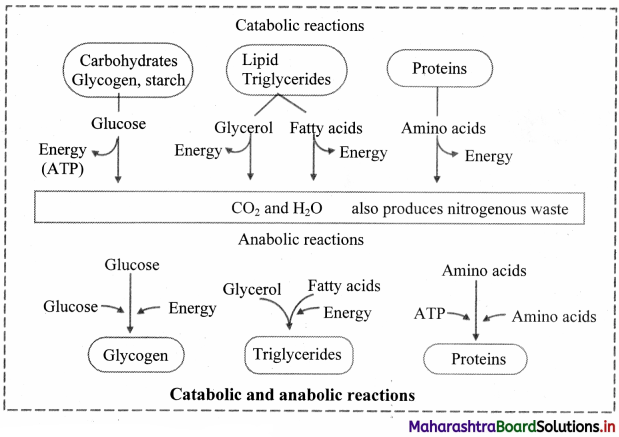
Draw a flowchart showing catabolic and anabolic reactions.
Answer:
Question 42.
Write a short note on secondary metabolites.
Answer:
- Secondary metabolites are small organic molecules produced by organisms that are not essential for their growth, development and reproduction.
- Several types of bacteria, fungi and plants produce secondary metabolites.
- Secondary metabolites can be classified on the basis of chemical structure (e.g. SMs containing rings, sugar), composition (with or without nitrogen), their solubility in various solvents, or the pathway by which they are synthesized (e.g. phenylpropanoid produces tannins).
- A simple way of classifying secondary metabolites includes three main groups such as:
- Terpenes: Made from mevalonic acid that is composed mainly of carbon and hydrogen
- Phenolics: Made from simple sugars containing benzene rings, hydrogen and oxygen.
- Nitrogen-containing compounds: Extremely diverse class may also contain sulphur.
![]()
Question 43.
Fill in the blanks.
- Living organism have _________ as the basic structural and functional unit.
- The cells have _______ containing numerous chemical molecules, the biomolecules.
- ________ are used very quickly by cells but if a cell is not in need of all the energy released immediately then it may get stored.
- By ________ reaction monosaccharide is converted to disaccharide.
- The balance between catabolism and anabolism maintain _______ in the cell as well as in the whole body.
Answer:
- Cell
- Protoplasm
- monosaccharides
- Condensation reaction
- Homeostasis
Question 44.
Apply Your Knowledge:
Question 1.
While performing an experiment, to understand effect of pH on enzyme activity, a student prepared
solution of varied pH. He observed that enzyme activity is maximum at a particular range of pH.
What is the reason for its maximum activity at a particular range of pH? What would be the effect on enzyme activity if strong acid or strong base is added?
Answer:
The enzymes are highly specific to pH and remain active within particular range of pH only. Hence, exhibit maximum activity only at particular range of pH. When strong acid or strong base is added in the reaction the enzyme activity is inhibited as most of the enzymes are denatured.
Question 2.
When a compound ‘x’ is added to a chemical solution containing enzyme and substrate, the enzymatic activity stops. What could be the nature of compound ‘x’?
Answer:
Compound ‘x’ could be either competitive or non-competitive inhibitor.
Question 45.
Quick Review
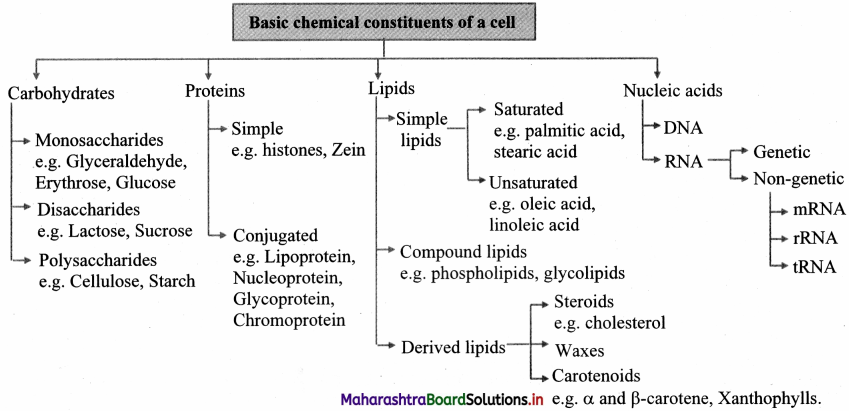
Question 46.
Exercise:
Question 1.
Draw a flow chart of biomolecules in living system.
Answer:
Refer Quick Review
![]()
Question 2.
Explain what is biochemistry?
Answer:
1. Biochemistry is biological chemistry that provides us the idea of the chemistry of living organisms and molecular basis for changes taking place in plants, animals and microbial cells.
2. It develops the foundation for understanding all biological processes and communication within and between cells as well as chemical basis of inheritance and diseases in animals and plants.
Question 3.
Mention the basic macromolecules present in the living organism.
Answer:
Polysaccharides (carbohydrate) polymer of monosaccharide, polypeptides (proteins) polymer of amino acids and polynucleotides (nucleic acids) polymer of nucleotides are the three basic macromolecule present in the living organisms.
Question 4.
Write a note on monosaccharides.
Answer:
Monosaccharides:
a. Monosaccharides are the simplest sugars having crystalline structure, sweet taste and soluble in water.
b. They cannot be further hydrolyzed into smaller molecules.
c. They are the building blocks or monomers of complex carbohydrates.
d. They have the general molecular formula (CH20)n, where n can be 3, 4, 5, 6 and 7.
e. They can be classified as triose, tetrose, pentose, etc.
f. Monosaccharides containing the aldehyde (-CHO) group are classified as aldoses e.g. glucose, xylose, and those with a ketone(-C=0) group are classified as ketoses. E.g. ribulose, fructose.
Question 5.
Explain the absorption of disaccharides through the cell membrane.
Answer:
1. Disaccharides are soluble in water but they are too big to pass through the cell membrane by diffusion.
2. They are broken down in the small intestine during digestion.
3. Thus, formed monosaccharides then pass into the blood and through cell membranes into the cells.
Question 6.
Draw the structure of amylose.
Answer:
Starch:
a. Starch is a stored food in the plants.
b. Starch contains two types of glucose polymer: amylose and amylopectin.
c. Both are made from a-glucose.
d. Amylose is an unbranched polymer of a-glucose.
e. The molecules coil into a helical structure.
f. It foims a colloidal suspension in hot water.
g. Amylopectin is a branched polymer of a-glucose.
h. It is completely insoluble in water.
Question 7.
Write the significance of carbohydrates.
Answer:
Significances of carbohydrates are as follows:
- Carbohydrates provide energy for metabolism.
- Glucose is the main substrate for ATP synthesis.
- Lactose, a disaccharide present in the milk provides energy to babies.
- Polysaccharide serves as a structural component of cell membrane, cell wall and reserved food as starch and glycogen.
![]()
Question 8.
What is glycosidic bond?
Answer:
Oligosaccharides:
a. A carbohydrate polymer comprising of two to six monosaccharide molecules is called oligosaccharide.
b. They are linked together by glycosidic bond.
c. They are classified on the basis of monosaccharide units:
Disaccharides: These are the sugars containing two monosaccharide units and can be further hydrolysed into smaller components. E.g.: Sucrose, maltose, lactose, etc.
Trisaccharides: These contain three monomers. E.g. Raffmose.
Tetrasaccharides: These contain four monomers. E.g.: Stachyose.
Glycosidic bond:
a. Glycosidic bond is a covalent bond that forms a linkage between two monosaccharides by a dehydration reaction.
b. It is formed when a hydroxyl group of one sugar reacts with the anomeric carbon of the other.
c. Glycosidic bonds are readily hydrolyzed by acid but resist cleavage by base.
d. There are two types of glycosidic bonds: a-glycosidic bond and P-glycosidic bond.
Question 9.
What are saturated fatty acids?
Answer:
1. Saturated fatty acids: They contain single chain of carbon atoms with single bonds.
E.g. Palmitic acid, stearic acid
2. Unsaturated fatty acids: They contain one or more double bonds between the carbon atoms of the hydrocarbon chain.
a. Simple lipids: These are esters of fatty acids with various alcohols.
E.g. Fats, wax.
b. Compound lipids: These are ester of fatty acids containing other groups like phosphate (Phospholipids), sugar (glycolipids), etc.
E.g. Lecithin
c. Sterols: They are derived lipids. They are composed of fused hydrocarbon rings (steroid nucleus) and a long hydrocarbon side chain.
E.g. Cholesterol, phytosterols.
Question 10.
Write a note on simple lipids.
Answer:
Lipids are classified into three main types:
Simple lipids:
a. These are esters of fatty acids with various alcohols. Fats and waxes are simple lipids.
b. Fats are esters of fatty acids with glycerol (CH2OH-CHOH-CH2OH).
c. Triglycerides are three molecules of fatty acids and one molecule of glycerol.
d. Unsaturated fats are liquid at room temperature and are called oils. Unsaturated fatty acids are hydrogenated to produce fats e.g. Vanaspati ghee.
Question 11.
Write a note on derived lipids.
Answer:
Derived Lipids:
a. They are composed of fused hydrocarbon rings (steroid nucleus) and a long hydrocarbon side chain.
b. One of the most common sterols is cholesterol.
Biological significance:
a. It is widely distributed in all cells of the animal body, but particularly in nervous tissue.
b. Cholesterol exists either free or as cholesterol ester.
c. Adrenocorticoids, sex hormones (progesterone, testosterone) and vitamin D are synthesized from cholesterol.
d. Cholesterol is not found in plants.
e. Sterols exist as phytosterols in plants.
f. Yam Plant (Dioscorea) produces a steroid compound called diosgenin. It is used in the manufacture of antifertility pills, i.e. birth control pills.
![]()
Question 12.
What are compound lipids? Mention their biological significance.
Answer:
Compound lipids:
a. These are ester of fatty acids containing other groups like phosphate (Phospholipids), sugar (glycolipids), etc.
b. They contain a molecule of glycerol, two molecules of fatty acids and a phosphate group or simple sugar.
c. Some phospholipids such as lecithin also have a nitrogenous compound attached to the phosphate group.
d. Phospholipids have both hydrophilic polar groups (phosphate and nitrogenous group) and hydrophobic non-polar groups (hydrocarbon chains of fatty acids).
e. Glycolipids contain glycerol, fatty acids, simple sugars such as galactose. They are also called cerebrosides.
Biological significance:
a. Phospholipids contribute in the formation of cell membrane.
b. Large amounts of glycolipids are found in the brain white matter and myelin sheath.
Question 13.
Explain the classification of proteins based on their chemical composition.
Answer:
On the basis of structure, proteins are classified into three categories:
1. Simple proteins:
a. Simple proteins on hydrolysis yield only amino acids.
b. These are soluble in one or more solvents.
c. Simple proteins may be soluble in water.
d. Histones of nucleoproteins are soluble in water.
e. Globular molecules of histones are not coagulated by heat.
f. Albumins are also soluble in water but they get coagulated on heating.
g. Albumins are widely distributed e.g. egg albumin, serum albumin and legumelin of pulses are albumins.
Importance: They are involved in structural components; they also act as a storage kind of protein.
Some are associated with nucleic acids in nucleoproteins of cell.
2. Conjugated proteins:
a. Conjugated proteins consist of a simple protein united with some non-protein substance.
b. The non-protein group is called prosthetic group e.g. haemoglobin.
c. Globin is the protein and the iron containing pigment haem is the prosthetic group.
d. Similarly, nucleoproteins have nucleic acids.
e. Proteins are classified as glycoproteins and mucoproteins.
f. Mucoproteins are carbohydrate-protein complexes e.g. mucin of saliva and heparin of blood.
g. Lipoproteins are lipid-protein complexes e.g. conjugate protein found in brain, plasma membrane, milk etc. Importance: They are involved in structural components of cell membranes and organelles.
They also act as a transporter.
Some conjugated proteins are important in electron transport chain in respiration.
3. Derived proteins:
a. These proteins are not found in nature as such.
b. These proteins are derived from native protein molecules on hydrolysis.
c. Metaproteins, peptones are derived proteins.
Importance: They act as a precursor for many molecules which are essential for life.
Question 14.
What is peptide bond? Explain its formation.
Answer:
1. The covalent bond that links the two amino acids is called a peptide bond.
2. Peptide bond is formed by condensation reaction.
Question 15.
Mention the examples of simple proteins and write their significance.
Answer:
Examples of simple proteins are: E.g.: Albumins and histones.
Significance:
1. Albumin:
a. % It is the main protein in the blood.
b. It maintains the pressure in the blood vessels.
c. It helps in transportation of substances like hormone and drugs in the body.
2. Histones:
a. It is the chief protein of chromatin.
b. They are involved in packaging of DNA into structural units called nucleosomes.
![]()
Question 16.
What is nucleotide?
Answer:
Nucleotide is a unit which consists of a sugar, phosphate and a base. Nucleotides are basic units of nucleic acids.
Question 17.
Write a note on structure of DNA molecule proposed by Watson and Crick.
Answer:
1. DNA is a long chain made up of alternate sugar and phosphate groups. The sugar present in DNA is always a deoxyribose attached to a phosphate group. So, it forms a regular, repeating phosphate sugar sequence.
2. A base is attached to sugar -phosphate chain. Together this unit which consist of sugar, phosphate and a base is called nucleotide.
3. The nitrogenous base and a sugar of a nucleotide form a molecule called nucleoside. It lacks phosphate group. Four types of nucleoside are found in DNA molecule.
4. In a nucleoside, nitrogenous base is attached to the first carbon atom (C-1) of the sugar and when a phosphate group gets attached with that of the carbon (C-5) atom of the sugar molecule a nucleotide molecule is formed.
5. A single strand of DNA consists of several thousands of nucleotides one above the other.
6. The phosphate group of the lower nucleotide attached with the 5th carbon atom of the deoxyribose sugar forms phospho-di-ester bond with that of the 3rd carbon atom of the deoxyribose sugar of the nucleotide placed just above it.
7. Single long chain of polynucleotides of DNA consists of one end with sugar molecules not connected with another nucleotide having C-3 carbon which is not connected with phosphate group, similarly the other end having C-5 of the sugar is not connected with any phosphate group. These two ends of the polynucleotide chain are called as 3′ and 5′ ends respectively.
8. The single polynucleotide strand of DNA is not straight but helical in shape.
9. The DNA molecule consists of such two helical polynucleotide chains which are complementary to each other.
10. The two complementary polynucleotide chains of DNA are held together by the weak hydrogen bonds.
11. Adenine always pairs with thymine, and guanine with cytosine (a pyrimidine with a purine).
12. Adenine-thymine pair consists of two hydrogen bonds and guanine-cytosine pair consists of three hydrogen bonds (Thus, if the sequence of bases of a polynucleotide chain is known, that of the other can be determined).
- According to Watson and Crick, DNA molecule consists of two strands twisted around each other in the form of a double helix.
- The two strands i.e. polynucleotide chains are supposed to be in opposite direction so end of one chain having 3′ lies beside the 5′ end of the other.
- One turn of the double helix of the DNA measures about 34A.
- It consists paired nucleotides and the distance between two neighboring pair nucleotides is 3.4A.
- The diameter of the DNA molecule has been found be 20A.
Question 18.
What is the function of ribosomal RNA?
Answer:
Ribosomal RNA (rRNA):
a. rRNA was discovered by Kurland in 1960.
b. It forms 50-60% part of ribosomes.
c. It accounts 80-90% of the cellular RNA.
d. It is synthesized in nucleus.
e. It gets coiled at various places due to intrachain complementary base pairing.
Role of ribosomal RNA: It provides proper binding site for m-RNA during protein synthesis.
Question 19.
Write a short note on m-RNA.
Answer:
Messenger RNA (mRNA):
a. It is a linear polynucleotide.
b. It accounts 3% of cellular RNA.
c. Its molecular weight is several million. , d. mRNA molecule carrying information to form a complete polypeptide chain is called cistron.
e. Size of mRNA is related to the size of message it contains.
f. Synthesis of mRNA begins at 5’ end of DNA strand and terminates at 3’ end.
Role of messenger RNA:
It carries genetic information from DNA to ribosomes, which are the sites of protein synthesis.
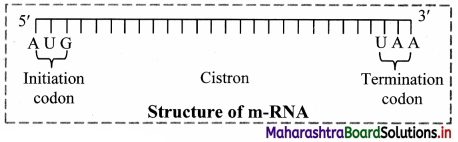
Question 20.
Write a note on types of non-genetic RNA.
Answer:
There are three types of cellular RNAs:
1. messenger RNA (mRNA),
2. ribosomal RNA (rRNA),
3. transfer RNA (tRNA). ‘
1. Messenger RNA (mRNA):
a. It is a linear polynucleotide.
b. It accounts 3% of cellular RNA.
c. Its molecular weight is several million. , d. mRNA molecule carrying information to form a complete polypeptide chain is called cistron.
e. Size of mRNA is related to the size of message it contains.
f. Synthesis of mRNA begins at 5’ end of DNA strand and terminates at 3’ end.
Role of messenger RNA:
It carries genetic information from DNA to ribosomes, which are the sites of protein synthesis.
2. Ribosomal RNA (rRNA):
a. rRNA was discovered by Kurland in 1960.
b. It forms 50-60% part of ribosomes.
c. It accounts 80-90% of the cellular RNA.
d. It is synthesized in nucleus.
e. It gets coiled at various places due to intrachain complementary base pairing.
Role of ribosomal RNA: It provides proper binding site for m-RNA during protein synthesis.
3. Transfer RNA (tRNA):
a. These molecules are much smaller consisting of 70-80 nucleotides.
b. Due to presence of complementary base pairing at various places, it is shaped like clover-leaf.
c. Each tRNA can pick up particular amino acid.
d. Following four parts can be recognized on tRNA
1. DHU arm (Dihydroxyuracil loop/ amino acid recognition site
2. Amino acid binding site
3. Anticodon loop / codon recognition site
4. Ribosome recognition site.
e. In the anticodon loop of tRNA, three unpaired nucleotides are present called as anticodon which pair with codon present on mRNA.
f. The specific amino acids are attached at the 3’ end in acceptor stem of clover leaf of tRNA.
Role of transfer RNA: It helps in elongation of polypeptide chain during the process called translation.
![]()
Question 21.
What are co-factors? Give examples.
Answer:
- Enzymes require certain inorganic ions for their activity.
- The inorganic ions which are loosely attached to the protein part are called co-factors.
- E.g. Magnesium, copper, zinc, iron, manganese etc.
[Note: Apoenzyme (protein part) and co-factor together form a complete catalytically active enzyme which is known as holoenzyme. The co-factors are of two types; metal ions and coenzymes. A coenzyme or metal ion that is very tightly or even covalently bound to the enzyme protein is known as a prosthetic group.]
Question 22.
Describe the important properties of enzymes.
Answer:
1. Proteinaceous Nature:
All enzymes are basically made up of protein.
2. Three-Dimensional conformation:
a. All enzymes have specific 3-dimensional conformation.
b. They have one or more active sites to which substrate (reactant) combines.
c. The points of active site where the substrate joins with the enzyme is called substrate binding site.
3. Catalytic property:
a. Enzymes are like inorganic catalysts and influence the speed of biochemical reactions but themselves remain unchanged.
b. After completion of the reaction and release of the product they remain active to catalyze again.
c. A small quantity of enzymes can catalyze the transformation of a very large quantity of the substrate
into an end product.
d. For example, sucrase can hydrolyze 100000 times of sucrose as compared with its own weight.
4. Specificity of action:
a. The ability of an enzyme to catalyze one specific reaction and essentially no other is perhaps its most significant property. Each enzyme acts upon a specific substrate or a specific group of substrates.
b. Enzymes are very sensitive to temperature and pH.
c. Each enzyme exhibits its highest activity at a specific pH i.e. optimum pH.
d. Any increase or decrease in pH causes decline in enzyme activity e.g. enzyme pepsin (secreted in stomach)shows highest activity at an optimum pH of 2 (acidic)
5. Temperature:
a. Enzymes are destroyed at higher temperature of 60-70°C or below, they are not destroyed but become inactive.
b. This inactive state is temporary and the enzyme can become active at suitable temperature.
c. Most of the enzymes work at an optimum temperature between 20°C and 35°C.
There are two types of models:
1. Lock and Key model:
a. Lock and Key model was first postulated in 1894 by Emil Fischer.
b. This model explains the specific action of an enzyme with a single substrate.
c. In this model, lock is the enzyme and key is the substrate.
d. The correctly sized key (substrate) fits into the key hole (active site) of the lock (enzyme).
2. Induced Fit model (Flexible Model):
a. Induced Fit model was first proposed in 1959 by Koshland.
b. This model states that approach of a substrate induces a conformational change in the enzyme.
c. It is the more accepted model to understand mode of action of enzyme.
d. The induced fit model shows that enzymes are rather flexible structures in which the active site continually reshapes by its interactions with the substrate until the time the substrate is completely bound to it.
e. It is also the point at which the final form and shape of the enzyme is determined.
[Note: Temperature is a factor affecting enzyme activity and not a property of enzyme.]
![]()
Question 23.
Explain the classification enzymes and mention the example of each class.
Answer:
1. Enzymes are biological macromolecules which act as a catalyst and accelerates the reaction in the body.
2. Enzymes are classified into six classes:
a. Oxidoreductases: These enzymes catalyze oxidation and reduction reactions by the transfer of hydrogen and/or oxygen, e.g. alcohol dehydrogenase
b. Transferases: These enzymes catalyse the transfer of certain groups between two molecules, e.g. glucokinase
c. Hydrolases: These enzymes catalyse hydrolytic reactions. This class includes amylases, proteases, lipases etc. e.g. Sucrase
d. Lyases: These enzymes are involved in elimination reactions resulting in the removal of a group of atoms from substrate molecule to leave a double bond. It includes aldolases, decarboxylases, and dehydratases, e.g. fumarate hydratase.
e. Isomerases: These enzymes catalyze structural rearrangements within a molecule. Their nomenclature is based on the type of isomerism. Thus, these enzymes are identified as racemases, epimerases, isomerases, mutases, e.g. xylose isomerase.
f. Ligases or Synthetases: These are the enzymes which catalyze the covalent linkage of the molecules utilizing the energy obtained from hydrolysis of an energy-rich compound like ATP, GTP e.g. glutathione synthetase, Pyruvate carboxylase.
Question 24.
Enlist the factors affecting the activity of enzymes.
Answer:
The factors affecting the enzyme activity are as follows:
1. Concentration of substrate:
a. Increase in the substrate concentration gradually increases the velocity of enzyme activity within the limited range of substrate levels.
b. A rectangular hyperbola is obtained when velocity is plotted against the substrate concentration.
c. Three distinct phases (A, B and C) of the reaction are observed in the graph.
Where V = Measured velocity, Vmax = Maximum velocity, S = Substrate concentration,
Km = Michaelis-Menten constant.
d. Km or the Michaelis-Menten constant is defined as the substrate concentration (expressed in moles/lit) to produce half of maximum velocity in an enzyme catalyzed reaction.
e. It indicates that half of the enzyme molecules (i.e. 50%) are bound with the substrate molecules when the substrate concentration equals the Km value.
f. Km value is a constant and a characteristic feature of a given enzyme.
g. It is a representative for measuring the strength of ES complex.
h. A low Km value indicates a strong affinity between enzyme and substrate, whereas a high Km value reflects a weak affinity between them.
i. For majority of enzymes, the Km values are in the range of 10-5 to 10-2 moles.
2. Enzyme Concentration:
a. The rate of an enzymatic reaction is directly proportional to the concentration of the substrate.
b. The rate of reaction is also directly proportional to the square root of the concentration of enzymes.
c. It means that the rate of reaction also increases with the increasing concentration of enzyme and the rate of reaction can also decrease by decreasing the concentration of enzyme.
3. Temperature:
a. The temperature at which the enzymes show maximum activity is called Optimum temperature.
b. The rate of chemical reaction is increased by a rise in temperature but this is true only over a limited range of temperature.
c. Enzymes rapidly denature at temperature above 40°C.
d. The activity of enzymes is reduced at low temperature.
e. The enzymatic reaction occurs best at or around 37°C which is the average normal body temperature in homeotherms.
4. Effect of pH:
a. The pH at which an enzyme catalyzes the reaction at the maximum rate is known as optimum pH.
b. The enzyme cannot perform its function beyond the range of its pH value.
5. Other substances:
a. The enzyme action is also increased or decreased in the presence of some other substances such as co-enzymes, activators and inhibitors.
b. Most of the enzymes are combination of a co-enzyme and an apo-enzyme.
c. Activators are the inorganic substances which increase the enzyme activity.
d. Inhibitor is the substance which reduces the enzyme activity.
![]()
Question 25.
With the help of lock and key theory explain the mechanism of enzyme action.
Answer:
1. Proteinaceous Nature:
All enzymes are basically made up of protein.
2. Three-Dimensional conformation:
a. All enzymes have specific 3-dimensional conformation.
b. They have one or more active sites to which substrate (reactant) combines.
c. The points of active site where the substrate joins with the enzyme is called substrate binding site.
3. Catalytic property:
a. Enzymes are like inorganic catalysts and influence the speed of biochemical reactions but themselves remain unchanged.
b. After completion of the reaction and release of the product they remain active to catalyze again.
c. A small quantity of enzymes can catalyze the transformation of a very large quantity of the substrate
into an end product.
d. For example, sucrase can hydrolyze 100000 times of sucrose as compared with its own weight.
4. Specificity of action:
a. The ability of an enzyme to catalyze one specific reaction and essentially no other is perhaps its most significant property. Each enzyme acts upon a specific substrate or a specific group of substrates.
b. Enzymes are very sensitive to temperature and pH.
c. Each enzyme exhibits its highest activity at a specific pH i.e. optimum pH.
d. Any increase or decrease in pH causes decline in enzyme activity e.g. enzyme pepsin (secreted in stomach)shows highest activity at an optimum pH of 2 (acidic)
5. Temperature:
a. Enzymes are destroyed at higher temperature of 60-70°C or below, they are not destroyed but become inactive.
b. This inactive state is temporary and the enzyme can become active at suitable temperature.
c. Most of the enzymes work at an optimum temperature between 20°C and 35°C.
There are two types of models:
1. Lock and Key model:
a. Lock and Key model was first postulated in 1894 by Emil Fischer.
b. This model explains the specific action of an enzyme with a single substrate.
c. In this model, lock is the enzyme and key is the substrate.
d. The correctly sized key (substrate) fits into the key hole (active site) of the lock (enzyme).
Question 26.
With the help of suitable examples give any three classes of enzymes.
Answer:
a. Oxidoreductases: These enzymes catalyze oxidation and reduction reactions by the transfer of hydrogen and/or oxygen, e.g. alcohol dehydrogenase
b. Transferases: These enzymes catalyse the transfer of certain groups between two molecules, e.g. glucokinase
c. Hydrolases: These enzymes catalyse hydrolytic reactions. This class includes amylases, proteases, lipases etc. e.g. Sucrase
Question 27.
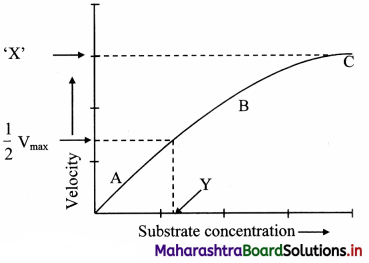
Following graph represents the effect of substrate concentration on enzyme activity. Identify ‘X’ and ‘Y’ Write proper explanation of the process.
Answer:
The factors affecting the enzyme activity are as follows:
1. Concentration of substrate:
a. Increase in the substrate concentration gradually increases the velocity of enzyme activity within the limited range of substrate levels.
b. A rectangular hyperbola is obtained when velocity is plotted against the substrate concentration.
c. Three distinct phases (A, B and C) of the reaction are observed in the graph.
Where V = Measured velocity, Vmax = Maximum velocity, S = Substrate concentration,
Km = Michaelis-Menten constant.
d. Km or the Michaelis-Menten constant is defined as the substrate concentration (expressed in moles/lit) to produce half of maximum velocity in an enzyme catalyzed reaction.
e. It indicates that half of the enzyme molecules (i.e. 50%) are bound with the substrate molecules when the substrate concentration equals the Km value.
f. Km value is a constant and a characteristic feature of a given enzyme.
g. It is a representative for measuring the strength of ES complex.
h. A low Km value indicates a strong affinity between enzyme and substrate, whereas a high Km value reflects a weak affinity between them.
i. For majority of enzymes, the Km values are in the range of 10-5 to 10-2 moles.
![]()
Question 28.
Explain the concept of metabolism.
Answer:
- Metabolism is the sum of the chemical reactions that take place within each cell of a living organism and provide energy for vital processes and for synthesizing new organic material.
- It involves continuous process of breakdown and synthesis of biomolecules through chemical reactions.
- Each of the metabolic reaction results in a transformation of biomolecules.
- Most of these metabolic reactions do not occur in isolation but are always linked with some other reactions.
- In cells, metabolism involves two following types of pathways:
a. Catabolic pathways
This involves formation of simpler structure from a complex biomolecule.
For e.g. when we eat wheat, bread or chapati, our gastrointestinal tract digests (hydrolyses) the starch to glucose units with help of enzymes and releases energy in form of ATP (Adenosine triphosphate).
b. Anabolic pathway
It is also called as biosynthetic pathway that involves formation of a more complex biomolecules from a simpler structure
Fone.g., synthesis of glycogen from glucose and protein from amino acids. These pathways consume energy.
Question 29.
Distinguish between Catabolic pathways and anabolic pathways.
Answer:
In cells, metabolism involves two following types of pathways:
a. Catabolic pathways
This involves formation of simpler structure from a complex biomolecule.
For e.g. when we eat wheat, bread or chapati, our gastrointestinal tract digests (hydrolyses) the starch to glucose units with help of enzymes and releases energy in form of ATP (Adenosine triphosphate).
b. Anabolic pathway
It is also called as biosynthetic pathway that involves formation of a more complex biomolecules from a simpler structure
Fone.g., synthesis of glycogen from glucose and protein from amino acids. These pathways consume energy.
Question 30.
Write the application of secondary metabolites.
Answer:
- Drugs developed from secondary metabolites have been used to treat infectious diseases, cancer, hypertension and inflammation.
- Morphine, the first alkaloid isolated from Papaver somniferum is used as pain reliver and cough suppressant.
- Secondary metabolites like alkaloids, nicotine, cocaine and the terpenes, cannabinol are widely used for recreation and stimulation.
- Flavours of secondary metabolites improve our food preferences.
- Tannins are added to wines and chocolate for improving astringency.
- Since most secondary metabolites have antibiotic property, they are also used as food preservatives.
- Glucosinolates is a secondary metabolite which is naturally present in cabbage imparts a characteristic flavour and aroma because of nitrogen and sulphur-containing chemicals. It also offers protection to these plants from many pests.
Question 31.
Explain the formation of metabolic pool.
Answer:
- Metabolism is the sum of the chemical reactions that take place within each cell of a living organism and provide energy for vital processes and for synthesizing new’ organic material.
- Metabolic pool in the cell is formed due to glycolysis and Krebs cycle.
- The catabolic chemical reaction of glycolysis and Krebs cycle provides ATP and biomolecules. These biomolecules form the metabolic pool of the cell.
- These biomolecules can be utilized for synthesis of many important cellular components.
- The metabolites can be added or withdrawn from the pool according to the need of the cell.
Question 32.
Explain the concept of metabolic pool.
Answer:
1. Metabolic pool is the reservoir of biomolecules in the cell on which enzymes can act to produce useful products as per the need of the cell.
2. The concept of metabolic pool is significant in cell biology because it allows one type of molecule to change into another type E.g. Carbohydrates can be converted to fats and vice-versa.
![]()
Question 33.
Multiple Choice Questions:
Question 1.
Most common constituents of organic compounds found in organims are
(A) C, H, O, P
(B) C, H, O
(C) C, H, N, P
(D) C, H, O, N, P
Answer:
(B) C, H, O
Question 2.
Carbohydrates are composed of
(A) carbon
(B) hydrogen
(C) oxygen
(D) all of these
Answer:
(D) all of these
Question 3.
In which of the following, the ratio of hydrogen and oxygen atoms is 2:1?
(A) proteins
(B) fats
(C) oil
(D) carbohydrates
Answer:
(D) carbohydrates
Question 4.
Which of the following do not give smaller sugar units on hydrolysis?
(A) Monosaccharides
(B) Disaccharides
(C) Polysaccharides
(D) Glycogen
Answer:
(A) Monosaccharides
Question 5.
The simplest monosaccharide made up of three carbons amongst the following is
(A) erythrose
(B) glucose
(C) glyceraldehyde
(D) ribose
Answer:
(C) glyceraldehyde
Question 6.
Deoxyribose sugar is an example of
(A) monosaccharide
(B) disaccharide
(C) polysaccharide
(D) simple protein
Answer:
(A) monosaccharide
Question 7.
Common examples of hexose sugar is/are
(A) glucose
(B) fructose
(C) erythrose
(D) both (A) and (B)
Answer:
(D) both (A) and (B)
![]()
Question 8.
If a compound contains 2 monosaccharides, then it is described as
(A) derived monosaccharide
(B) disaccharide
(C) polysaccharide
(D) pentose sugar
Answer:
(B) disaccharide
Question 9.
In a disaccharide, monomers are linked with each other through ________ bonds.
(A) peptide
(B) hydrogen
(C) glycosidic
(D) ester
Answer:
(C) glycosidic
Question 10.
A disaccharide that gives two molecules of glucose on hydrolysis is
(A) sucrose
(B) maltose
(C) lactose
(D) none of these
Answer:
(B) maltose
Question 11.
Sugar present in milk is
(A) fructose
(B) lactose
(C) galactose
(D) sucrose
Answer:
(B) lactose
Question 12.
Polysaccharides consist of
(A) two monosaccharide units
(B) eight monosaccharide units
(C) many monosaccharide units
(D) amino acids
Answer:
(C) many monosaccharide units
![]()
Question 13.
________ are water insoluble and small molecular weight compounds as compared to macromolecules.
(A) Lipids
(B) proteins
(C) carbohydrates
(D) nucleic acids.
Answer:
(A) Lipids
Question 14.
Simple lipids are esters of
(A) amino acids
(B) proteins
(C) phosphorus
(D) fatty acids with glycerol
Answer:
(D) fatty acids with glycerol
Question 15.
Fatty acids which do not contain double bond between carbon atoms are
(A) saturated fatty acids
(B) unsaturated fatty acids
(C) oleic and linoleic acids
(D) linoleic and linolenic acids
Answer:
(A) saturated fatty acids
Question 16.
Proteins are linear polymers of
(A) amino acids
(B) fatty acids
(C) monosaccharides
(D) nucleic acids
Answer:
(A) amino acids
Question 17.
Proteins are formed by the condensation of
(A) nucleic acids
(B) amino acids
(C) fatty acids
(D) carbohydrates
Answer:
(B) amino acids
Question 18.
Protein is
(A) micromolecule
(B) macromolecule
(C) soluble
(D) specific
Answer:
(B) macromolecule
![]()
Question 19.
Keratin is a ________ protein.
(A) transport
(B) protective
(C) structural
(D) storage
Answer:
(C) structural
Question 20.
A nucleotide contains
(A) sugar + phosphate
(B) N-base + phosphate
(C) sugar + nitrogenous base
(D) sugar + N-base + phosphate
Answer:
(D) sugar + N-base + phosphate
Question 21.
Nucleotides, the polymers of nucleic acid are joined together by __________ bond.
(A) Peptide
(B) Ester
(C) Phosphodiester
(D) Glycosidic
Answer:
(C) Phosphodiester
Question 22.
Find the odd one.
(A) Adenine
(B) Cytosine
(C) Thymine
(D) Uracil
Answer:
(D) Uracil
Question 23.
The two strands of DNA are
(A) similar in nature and complementary
(B) anti-parallel and complementary
(C) parallel and complementary
(D) basically, different in nature
Answer:
(B) anti-parallel and complementary
Question 24.
RNA is genetic material in
(A) bacteria
(B) cyanobacteria
(C) bacteriophages
(D) plant viruses
Answer:
(D) plant viruses
![]()
Question 25.
Which RNA is present in more amount in the cell?
(A) m-RNA
(B) t-RNA
(C) r-RNA
(D) not certain
Answer:
(C) r-RNA
Question 26.
Smallest RNA is
(A) t-RNA
(B) m-RNA
(C) r-RNA
(D) not specific
Answer:
(A) t-RNA
Question 27.
________ catalyze hydrolysis of ester, ether etc.
(A) Lyases
(B) Ligases
(C) Hydrolases
(D) Transferases
Answer:
(C) Hydrolases
Question 28.
_______ catalyze interconversions of geometric, optical and positional isomers.
(A) Transferases
(B) Ligases
(C) Oxidoreductase
(D) Isomerases
Answer:
(D) Isomerases
Question 29.
Metal cofactors are also known as?
(A) prosthetic group
(B) coenzyme
(C) activators
(D) inhibitors
Answer:
(C) activators
Question 30.
________ are also known as dehydrogenases.
(A) Oxidoreductases
(B) Ligases
(C) Lyases
(D) Transferases
Answer:
(A) Oxidoreductases
Question 31.
The enzyme functions best at temperature
(A) 30°C to 50°C
(B) 15°C to 25°C
(C) 20°C to 35°C
(D) 40°C to 50°C
Answer:
(C) 20°C to 35°C
![]()
Question 32.
As temperature changes from 30° to 45° C, the rate of enzyme activity will
(A) decrease
(B) increase
(C) first increase and then decrease
(D) first decrease and then increase
Answer:
(C) first increase and then decrease
Question 33.
Out of the following, which is not a property of enzymes?
(A) Specific in nature
(B) Proteinaceous
(C) Used up in reaction
(D) Increased rate of biochemical reaction
Answer:
(C) Used up in reaction
Question 34.
Majority of cellular enzymes function best at _______ PH.
(A) acidic
(B) basic
(C) neutral
(D) strong base
Answer:
(B) basic
Question 35.
The _______ action of enzyme with a substrate is explained by lock and key theory.
(A) relative
(B) specific
(C) random
(D) abstract
Answer:
(B) specific
Question 36.
Morphine, the first alkaloid isolated from ________
(A) Pisum sativum
(B) Hibiscus rosa sinensis
(C) Papaver somniferum
(D) Azadirachta indica
Answer:
(C) Papaver somniferum
![]()
Question 34.
Competitive Corner:
Question 1.
Prosthetic groups differ from co-enzymes in that –
(A) They can serve as co-factors in a number of enzyme – catalyzed reactions
(B) They require metal ions for their activity
(C) They (prosthetic groups) are tightly bound to apoenzymes
(D) Their association with apoenzymes is transient
Hint: Apoenzyme (protein part) and co-factor together form a complete catalytically active enzyme which is known as holoenzyme. The co-factors are of two types; metal ions and coenzymes. A coenzyme or metal ion that is very tightly or even covalently bound to the enzyme protein is known as a prosthetic group.
Answer:
(C) They (prosthetic groups) are tightly bound to apoenzymes
Question 2.
Consider the following statements:
1. Coenzyme or metal ion that is tightly bound to enzyme protein is called prosthetic group.
2. A complete catalytic active enzyme with its bound prosthetic group is called apoenzyme. Select the correct option.
(A) Both (i) and (ii) are false.
(B) (i) is false but (ii) is true.
(C) Both (i) and (ii) are true.
(D) (i) is true but (ii) is false.
Answer:
(D) (i) is true but (ii) is false.
Question 3.
Concanavalin A is:
(A) a lectin
(B) a pigment
(C) an alkaloid
(D) an essential oil
Answer:
(A) a lectin
Question 4.
Which one of the following carbohydrates is a heteropolysaccharide?
(A) Cellulose
(B) Starch
(C) Glycogen
(D) Hyaluronic acid
Answer:
(D) Hyaluronic acid
Question 5.
The two functional groups characteristic of sugars are
(A) Carbonyl and phosphate
(B) Carbonyl and methyl
(C) Hydroxyl and methyl
(D) Carbonyl and hydroxyl
Answer:
(D) Carbonyl and hydroxyl
![]()
Question 6.
Which one of the following statements is correct with reference to enzymes?
(A) Apoenzyme = Holoenzyme + coenzyme
(B) Holoenzyme = Apoenzyme + Coenzyme
(C) Coenzyme = Apoenzyme + Holoenzyme
(D) Holoenzyme = Coenzyme + Co-factor
Answer:
(B) Holoenzyme = Apoenzyme + Coenzyme
Question 7.
Which of the following are NOT polymeric?
(A) Nucleic acids
(B) Proteins
(C) Polysaccharides
(D) Lipids
Answer:
(D) Lipids