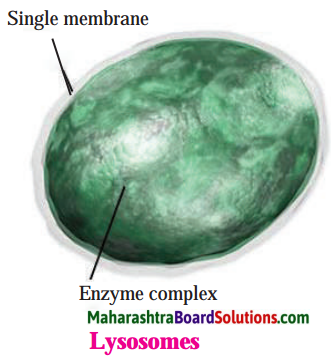
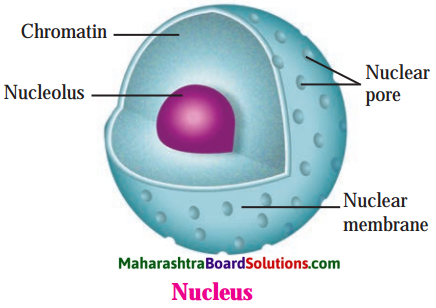
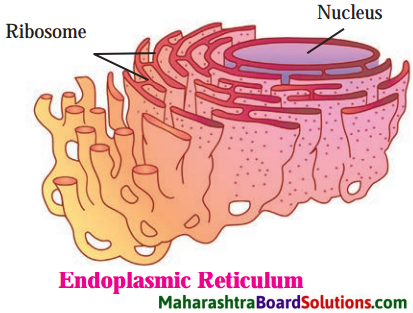
Balbharti Maharashtra State Board Class 8 Science Solutions Chapter 11 Human Body and Organ System Notes, Textbook Exercise Important Questions and Answers.
Maharashtra State Board Class 8 Science Solutions Chapter 11 Human Body and Organ System
Class 8 Science Chapter 11 Human Body and Organ System Textbook Questions and Answers
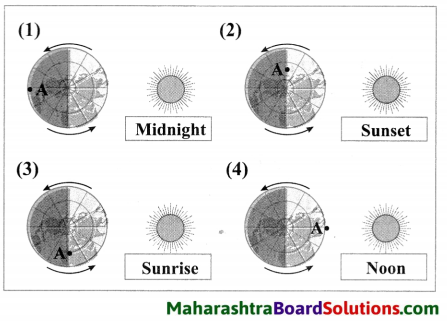
1. Find out my partner.
Question a.
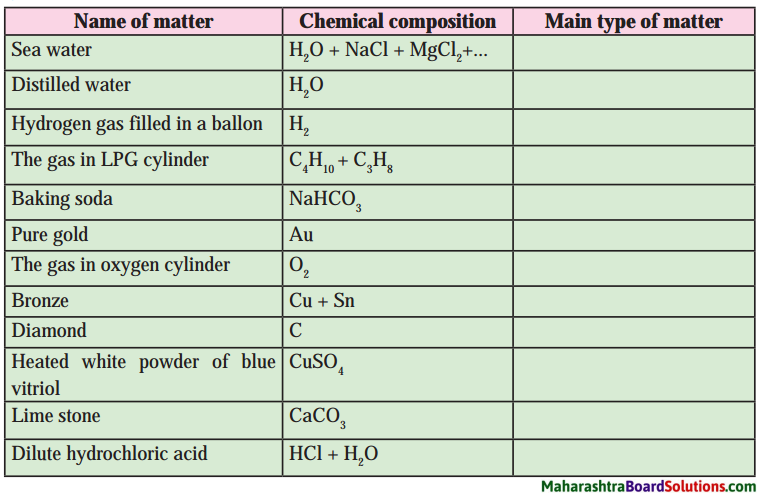
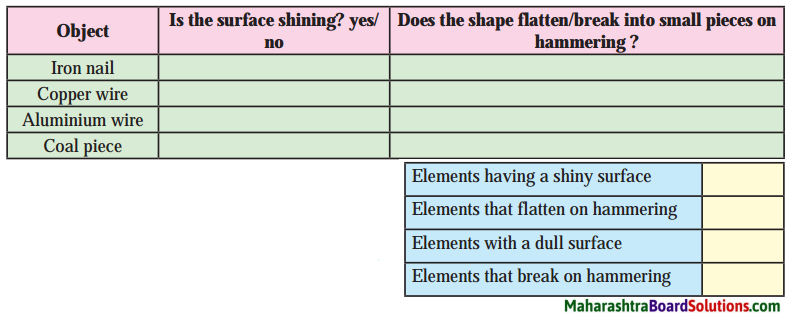
| Group ‘A’ | Group ‘B’ |
| 1. Heartbeats | a. 350 ml |
| 2. RBC | b. 7.4 |
| 3. WBC | c. 37° C |
| 4. Blood donation | d. 72 per min |
| 5. Normal body temperature | e. 50 – 60 lakh/mm3 |
| 6. pH of oxygenated blood | f. 5000 – 6000 per mm3 |
Answer:
| Group ‘A’ | Group ‘B’ |
| 1. Heartbeats | d. 72 per min |
| 2. RBC | e. 50 – 60 lakh/mm3 |
| 3. WBC | f. 5000 – 6000 per mm3 |
| 4. Blood donation | a. 350 ml |
| 5. Normal body temperature | c. 37° C |
| 6. pH of oxygenated blood | b. 7.4 |
![]()
2. Complete the following table.
Question a.
Answer:
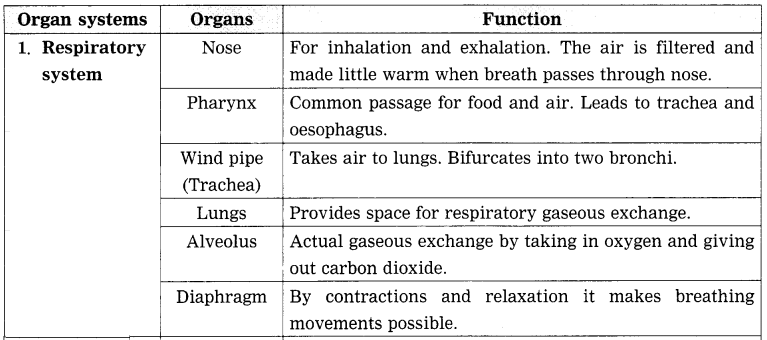
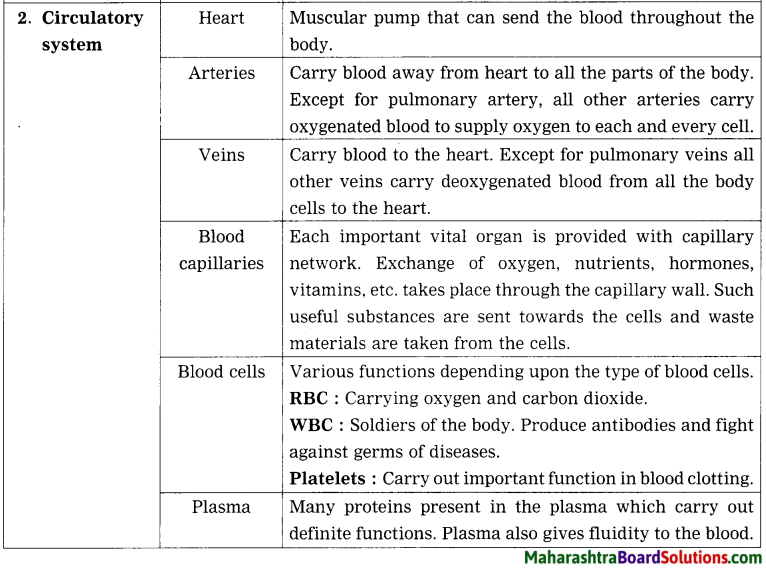
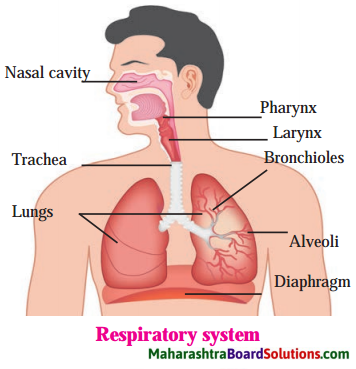
3. Draw neat and labeled diagrams.
Question a.
Respiratory system.
Answer:
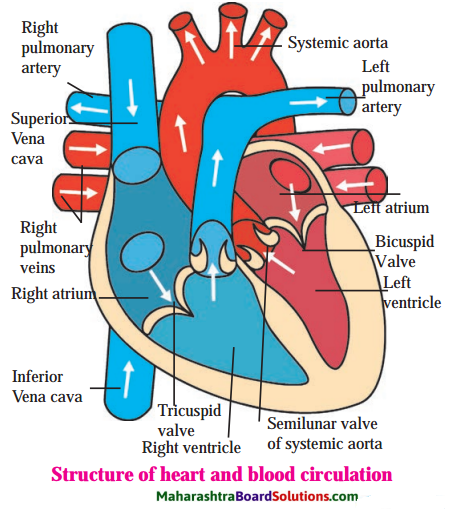
Question b.
Internal structure of heart.
Answer:
4. Explain with reasons.
Question a.
Human blood is red coloured.
Answer:
The red colour of human blood is due to hemoglobin which is a red coloured conjugated protein with iron that is present on the red blood cells. Therefore, it looks red.
Question b.
Upward and downward movement of diaphragm occurs consecutively.
Answer:
The breathing movements are possible due to contraction and relaxation of the diaphragm. The rib muscles also help in these movements. When the ribs rise and diaphragm is lowered at the same time, then there is a decrease in pressure on lungs.
This causes movement of air into the lungs at the time of inhalation. On the other hand, when ribs come back to their normal position and diaphragm is risen, then pressure on the lungs increases. This causes movement of the air out of the body through the nose in the form of exhalation. These movements are possible only due to consecutive upward and downward movement of the diaphragm.
![]() b
b
Question c.
Blood donation is considered to be superior of all donations.
Answer:
Blood cannot be manufactured by any artificial chemical process. The only way to obtain blood is by donations of blood from a live donor. Blood is needed at times of emergency. The life of person can be saved if timely blood transfusion is given to the needy victim or a patient. Since such donation can save a valuable human life, it is called superior of all donations.
Question d.
Person with ‘O’ blood group is considered as ‘universal donor’.
Answer:
Person with ‘O’ blood group does not have any antigen on his/her RBCs. The ‘O’ type blood thus cannot cause clotting reactions in the body of the recipients. Such persons with ‘O’ blood group can donate blood to any person having any blood group therefore they are considered as ‘universal donor’.
Question e.
Food must have limited amount of salts.
Answer:
More salt in diet means more sodium ions. These extra sodium salts cause rise in blood pressure. Such condition is called hypertension. This condition can be dangerous and fatal in some cases. Therefore, one must keep control over sodium content of the food.
![]()
5. Answer the following questions in your own words.
Question a.
Explain the functional correlation of circulatory system with respiratory, digestive and excretory system.
Answer:
1. Three systems viz. respiratory, digestive and circulatory always work in coordination.
2. Digestive system helps in breaking down complex food molecules into simple soluble nutrients at the end of the digestion process.
3. The soluble nutrients are absorbed in the circulating blood in the villi of the intestine.
4. The blood carries these nutrients to each cell during its circulation.
5. The respiratory system helps the oxygen from the air to be absorbed in the blood.
6. This process takes place in alveolus present in lungs. The oxygen is absorbed in the blood and through haemoglobin it is taken to every cell of the body. At the same time the unwanted carbon dioxide produced in each cell is given out in a process of gaseous exchange.
7. The soluble nutrients, and chiefly glucose is metabolized with the help of oxygen-producing energy. Thus, all the three systems bring about coordinated functions to keep the body alive.
![]()
Question b.
Explain the structure and function of human blood.
Answer:
I. Structure, i.e. components of the human blood: Human blood is a fluid connective tissue consisting of blood plasma and blood corpuscles suspended in it.
1. Plasma: Plasma is the fluid part of the blood which is pale yellow in colour. It is slightly alkaline in nature. It has 90-92% water, 6-8 % proteins and 1-2 % inorganic salts.
It contains proteins such as albumin, globulin, fibrinogen, etc. There are inorganic ions such as Ca, Na and K.
2. Blood cells:
a. Blood cells are mainly of three types, viz. RBCs, WBCs and blood platelets. They are produced in the red bone marrow.
b. RBCs are small, circular and enucleated cells. They are full of haemoglobin which is essential in transporting oxygen. RBCs are red blood cells which are 50 to 60 lakh per cubic millimetre. Their life span is 100 to 127 days.
c. WBCs are large, nucleated and colourless. They are of five subtypes, viz. neutrophils, basophils, eosinophils, monocytes and lymphocytes. They are 5 to 10 thousands per millimetre of blood.
d. Platelets are very small disc-shaped blood cells which are 2.5 to 4 lakh per cubic millimetre of blood.
II. Function of human blood:
1. Transport functions:
- Gases: Oxygen is carried via blood from lungs to cells in various parts of body and carbon dioxide from tissues to lungs.
- Nutrients: Simple nutrients like glucose, amino acids, fatty acids are taken up by blood from wall of alimentary canal and transported up to each cell in the body.
- Waste materials: Nitrogenous wastes like ammonia, urea, creatinine are released by tissues into blood which carries those to kidney for excretion.
- Enzymes and hormones: Blood transports the enzymes and hormones from the site of their production to the site of their action.
2. Protection: Antibodies are produced in the blood and they protect the body from microbes and other harmful particles.
3. Thermoregulation: Body temperature is maintained constant at 37 °C by vasodilation and vasoconstriction.
4. Maintaining the balance of minerals like Na, K in the body.
5. If bleeding occurs at the injury, platelets and a protein called fibrinogen of the blood form a clot and seal the injury.
6. Functions of blood cells:
- RBCs: With haemoglobin it carries out transport of respiratory gases.
- WBCs: Soldiers of the body. Produce antibodies and give immunity to body.
- Platelets: Help in blood clotting.
![]()
Question c.
Explain the importance and need of blood donation.
Answer:
Blood can never be synthesized artificially. There is no substitute for natural blood. Every healthy person possesses about 5 litres of blood in his or her body. In case of haemorrhage i.e. blood loss, the blood volume may reduce which can result into threat to life. Moreover, the loss of blood should be immediately taken care of, otherwise it may cost the life.
Therefore blood transfusion is very crucial in case of victims of accidents, patients of surgeries or mothers who suffer from blood loss during childbirth (parturition). Some diseases such as thalassemia, blood cancer, etc. also need regular transfusions. Therefore, blood is always needed in many such conditions. Blood donation is only option for such transfusions.
6. Explain the differences.
Question a.
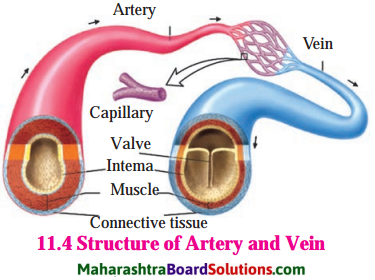
Arteries and veins.
Answer:
| Arteries | Veins |
| 1. Arteries carry blood away from the heart to the tissues of the body. | 1. Veins carry blood from the tissues of the body back to the heart. |
| 2. Arteries are located deeper within the body. | 2. Veins are usually located superficially beneath the surface of the skin. |
| 3. Arteries are thick walled | Veins are thin walled. |
| 4. Arteries do not have valves. | 4. Veins have valves. |
| 5. Arteries would generally remain open if blood flow stopped, due to their thick muscular layer. | 5. Veins would collapse if blood flow stops. |
| 6. Except pulmonary artery, all arteries carry oxygenated blood. | 6. Except pulmonary vein, all veins carry deoxygenated blood. |
| 7. Arteries are more muscular than veins, which helps in transporting blood that is full of oxygen efficiently to the tissues. | 7. Veins are less muscular than arteries, but contain valves to help keep blood flowing in the right direction, usually toward the heart. |
| 8. There is maximum blood pressure in the arteries. | 8. There is minimum blood pressure in the veins. |
![]()
Question b.
External and internal respiration.
Answer:
| External respiration | Internal respiration |
| 1. Intake of air from the outside into the body and release of air from the body to outside is called external respiration. | 1. Exchange of gases between cells and tissue fluid is called internal respiration. |
| 2. External respiration occurs between cells and the external environment. | 2. Internal respiration occurs only in the cells of the body. |
| 3. It involves processes of inspiration and expiration. | 3. It involves movement of O2 from blood into tissue fluid and movement of CO2 from tissue. |
| 4. External respiration involves breathing and gaseous exchange. | 4. Internal respiration involves neither breathing nor gaseous exchange. |
| 5. Oxygen combines with haemoglobin in external respiration. | 5. Chemical reactions occur in the cells to form energy. |
7. Which health parameters of blood donor should be checked?
Question a.
Which health parameters of blood donor should be checked?
Answer:
Blood donor should be healthy. He or she should have good haemoglobin’s content. The RBC and WBC count should also be normal. They should not carry any parasites in their blood such as malarial parasite or dengue virus. The donor should not be HIV positive or should not have any infectious diseases. He should not have any addictions such s drug-abuse or alcohol consumption.
![]()
8. Fill in the blanks using appropriate words given in the bracket.
(hemoglobin, alkaline, diaphragm, red bone marrow, acidic, voluntary, involuntary,)
Question a.
RBCs of the blood contain ……….., an iron compound.
Answer:
RBCs of the blood contain haemoglobin, an iron compound.
Question b.
……………….. is present between thoracic and abdominal cavity.
Answer:
Diaphragm is present between thoracic and abdominal cavity.
Question c.
Cardiac muscles are …………… .
Answer:
Cardiac muscles are involuntary.
Question d.
pH of oxygenated blood is …………… .
Answer:
pH of oxygenated blood is alkaline.
![]()
Question e.
Production of RBCs occurs in ……………… .
Answer:
Production of RBCs occurs in red bone marrow.
8. Find odd one out.
Question a.
A, O, K, AB, B.
Answer:
K (All others are blood groups.)
Question b.
Blood plasma, platelets, blood transfusion, blood corpuscles.
Answer:
Blood transfusion (All others are components of blood.)
Question c.
Trachea, alveoli, diaphragm, capillaries.
Answer:
Capillaries (All others are parts of respiratory system. Capillaries exist throughout the body.)
![]()
Question d.
Neutrophils, globulins, albumins, prothrombin.
Answer:
Neutrophils (All others are proteins present in the plasma.)
10. Read the following paragraph and identify the disease.
Today, her child became one and half year old. However, that child does not seem to be healthy and happy. It was continuously crying and gradually becoming weak. It has shortness of breath. Its nails have become blue.
Answer:
The heart of the child is not functioning properly. Bluish nails show lack of oxygen, thus the baby may be suffering also from respiratory problems.
11. Your neighboring uncle has been diagnosed with hypertension. What should he do to keep his blood pressure within normal range?
Question a.
Your neighboring uncle has been diagnosed with hypertension. What should he do to keep his blood pressure within normal range?
Answer:
Hypertension has many causes. Try to find out what is the exact cause. Is it lack of exercise, obesity, lots of fast and junk food consumption, over-intake of salt, mental tension, etc. He should visit a proper physician and take prescribed blood pressure control medicines. He should never miss a single tablet. He should avoid salty and preserved food. He should practice yoga and meditation. He should also undertake some stress- management techniques.
Project:
Question a.
Collect information about various modern treatments on heart diseases.
Class 8 Science Chapter 11 Human Body and Organ System Important Questions and Answers
Rewrite the statements after correcting them:
Question 1.
Cells need supply of insoluble nutrients and oxygen for the energy production.
Answer:
Cells need the supply of soluble nutrients and oxygen for the energy production.
![]()
Question 2.
Respiratory system and respiration begins with mouth.
Answer:
Respiratory system and respiration begins with nose.
Question 3.
A lung is present on either sides of heart in abdominal cavity.
Answer:
A lung is present on either sides of heart in thoracic cavity.
Question 4.
Continuous upward and downward movement of diaphragm is necessary to bring about the beating of heart.
Answer:
Continuous upward and downward movement of diaphragm is necessary to bring about the breathing.
Question 5.
Blood vessels which carry the blood away from heart are called veins.
Answer:
Blood vessels which carry the blood away from heart are called arteries.
![]()
Question 6.
William Harvey described the blood groups in man.
Answer:
William Harvey described the mechanism of circulation in the body.
Question 7.
Capillaries unite together to form the arteries.
Answer:
Capillaries unite together to form the veins.
Match the columns/Find out my partner:
Question 1.
| Group ‘A’ | Group ‘B’ |
| 1. William Harvey | a. Blood group AB. |
| 2. Carl Landsteiner | b. A, B, O blood group system. |
| 3. Decastello and Sturli | c. Mechanism of circulation of blood. |
Answer:
| Group ‘A’ | Group ‘B’ |
| 1. William Harvey | c. Mechanism of circulation of blood. |
| 2. Carl Landsteiner | b. A, B, O blood group system. |
| 3. Decastello and Sturli | a. Blood group AB. |
![]()
Complete the following table:
Question 1.
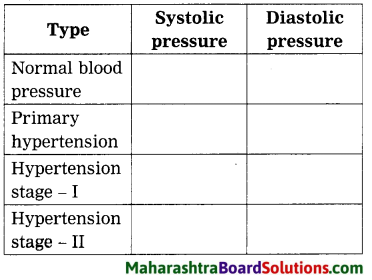
Answer:
| Type | Systolic pressure | Diastolic pressure |
| Normal blood pressure | 90 – 119 mmHg | 60 – 79 mmHg |
| Primary hypertension | 120 – 139 mmHg | 80 – 89 mmHg |
| Hypertension stage – I | 140 – 159 mmHg | 90 – 99 mmHg |
| Hypertension stage – II | > 160 mmHg | > 100 mm |
Define the following terms:
- Inhalation: Inhalation is taking in air through the nose from the surrounding
- Exhalation: Exhalation is giving out the air back to the outer environment.
- External respiration: The processes of inhalation and exhalation both together are called external respiration.
- Internal respiration: Exchange of gases between cells and tissue fluid is called internal respiration.
- Cellular respiration: Production of energy in the form of ATP from oxidation of glucose and other soluble nutrients is called cellular respiration.
- Thermoregulation: Maintenance of the body temperature to a constant level by performing vasoconstriction or vasodilation is called thermoregulation.
- Blood pressure: Pressure exerted by the flowing blood on the blood vessel wall is called blood pressure.
- Systolic pressure: The maximum blood pressure exerted by the flowing blood when the heart is contracting is called systolic pressure.
- Diastolic pressure: The minimum blood pressure exerted by the flowing blood when the heart is not contracting but receiving (i.e. relaxing) the blood is called diastolic pressure.
- Hypertension or High Blood Pressure: The blood pressure value of 140 – 159 mm Hg which is more than the normal blood pressure is called hypertension or high blood pressure.
- Sphygmomanometer: The instru¬ment used for measuring blood pressure is called sphygmomanometer.
- Hematology: The branch of medical science in which the blood, haemapoietic organs (organs that produce blood cells) and blood disorders are studied is called haematology.
![]()
Answer the following questions in one or two sentences each:
Question 1.
What are the heart sounds and why are they produced?
Answer:
There are two types of heart sounds, one is ‘lubb’ and other is ‘dub’. These are produced due to closure of the heart valves.
Question 2.
What is blood circulation?
Answer:
The process of pumping the blood to all the parts of the body and bringing it back again to the heart is called the blood circulation.
Question 3.
Name any four proteins present in the blood plasma.
Answer:
The proteins present in the blood plasma are albumin, globulins, fibrinogen and prothrombin.
Question 4.
Which inorganic ions control the function of muscles and nerves?
Answer:
Calcium, sodium and potassium control the functions of muscles and nerves.
![]()
Question 5.
What is the basis on which blood group is determined?
Answer:
The antigen present on the RBCs: and the antibodies present in the plasma of I the blood determine the type of blood group. The genes of parents are responsible for the type of blood group that the child inherits.
Question 6.
Under which conditions, blood is required for donation?
Answer:
Whenever there is hemorrhage, the patient requires blood. Such patients are accident victims, those who excessively bleed, women during childbirth (parturition) and for patients undergoing surgeries.
Question 7.
How much blood is collected from a person during donation?
Answer:
About 350 ml of blood is collected from a person during donation.
Question 8.
When is National Voluntary Blood Donation Day observed?
Answer:
National Voluntary Blood Donation Day is observed on 1st October every year.
Explain the differences:
Question 1.
Atria and ventricles:
Answer:
| Atria | Ventricles |
| 1. Atria are the upper chambers of the heart. | 1. Ventricles are the lower chambers of the heart. |
| 2. Atria are the smaller receiving chambers. | 2. Ventricles are the larger distributing chambers. |
| 3. Atria are thin walled chambers having lesser blood pressure. | 3. Ventricles are thick walled chambers with greater blood pressure. |
| 4. Atria do not have inlet valves. | 4. Ventricles have inlet valves. |
| 5. Right atrium receives deoxygenated blood from whole body through the inferior vena cava (lower body), superior vena cava (from upper body). It pumps blood into right ventricle. | 5. Right ventricle pumps deoxygenated blood received from right atrium to lungs for oxygenation which is known as pulmonary circulation. |
| 6. Left atrium receives oxygenated blood from lungs through pulmonary veins. It pumps blood into left ventricle. | 6. Left ventricle pumps oxygenated blood received from left atrium to whole body. This is called systemic circulation. |
![]()
Question 2.
RBCs and WBCs:
Answer:
| RBCs | WBCs |
| 1. RBCs are red in colour due to haemoglobin present in them. | 1. WBCs are colourless as there is no pigment in them. |
| 2. RBCs are produced in red bone marrow. | 2. WBCs are produced mostly in bone marrow. But they are also produced in lymph nodes, spleen, etc. |
| 3. RBCs are smaller in size and rounded in .shape. | 3. WBCs are larger in size and are of different shapes. |
| 4. RBCs have an average lifespan of 120 days. | 4. Life span of WBCs vary according to their role. They have a life span from a few days to 3 weeks. |
| 5. Normal RBC count is 50 – 60 lakh RBCs per cubic mm. of blood. | 5. Normal WBC count is 5 thousands to 10 thousands per cubic mm. of blood. |
| 6. Their function is to transport the respiratory gases (Oxygen and Carbon Dioxide). | 6. Their main function is to produce antibodies and fight against the infections. Thus they are called soldiers of the body. |
Give scientific reasons:
Question 1.
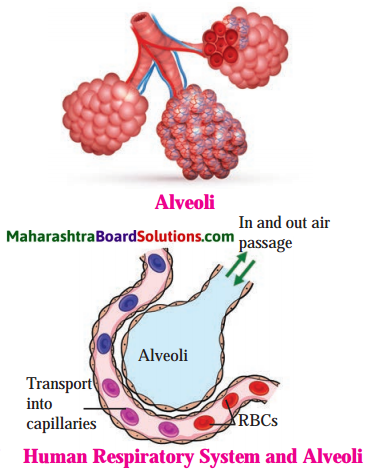
A very large number of alveoli is present in lungs, which are covered over by capillary network.
Answer:
Due to very large number of alveoli the surface area of the lungs is increased many a times for the gaseous exchange. The alveoli are covered over by capillary network for rapid gaseous exchange. The oxygen is taken in the body and at the same time carbon dioxide is given out of the body only by the gaseous exchange occurring at the alveolar surface.
![]()
Question 2.
Heart is covered by double layered pericardial membrane.
Answer:
Pericardium is the protective double membrane that covers the heart. In between the two layers of this membrane there is protective fluid. The pericardium and the fluid together protect the heart from friction and mechanical shock. Since heart is a vital organ, it is well protected by such pericardial membrane.
Question 3.
Veins are provided with valves.
Answer:
Valves prevent the backflow of the blood. Blood in the veins is not under great pressure so it is likely that it may flow back. But valves prevent such movements. Therefore they are provided with valves.
Write short notes on the following:
Question 1.
Cellular respiration:
Answer:
- During respiration, the glucose molecules along with some other soluble nutrients are slowly oxidized with the help of oxygen in each cell.
- In this process the energy is released in the form of ATP, CO2 and water vapours are produced.
- These products are not needed for the body and hence given out of the body in exhalation.
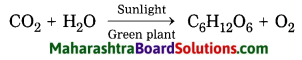
- This process of cellular respiration is shown by the following reaction:
C6H12O6 + 6O2 = 6CO2 + 6H2O + Energy (38ATP)
Question 2.
Lung:
Answer:
- Pair of lungs is the main respiratory organ in the human body. They are located in thoracic cavity.
- They are present on either sides of heart.
- Each lung has double layered pleural membranes.
- Trachea bifurcates into two bronchi. Each bronchus enters lung on its side and S divide and re-divide into fine bronchioles.
- At the end of each bronchiole there is alveolus. Alveolus is surrounded by capillary network.
- Each alveolus is extremely thin walled and hence gaseous exchange can occur through diffusion here. Due to thousands of alveoli, the lung surface is increased many a times.
- Deoxygenated blood coming from heart by pulmonary arteries is purified here in the lungs.
- It is mixed with oxygen due to gaseous exchange and returned back to the heart by pulmonary veins. Lungs thus continuously help in oxygenation of blood with the help of all of the alveoli.
![]()
Question 3.
Diaphragm:
Answer:
- Diaphragm is a muscular partition that divides the thoracic and abdominal cavity.
- Located at the base of thoracic cage, it is very important in breathing movements.
- Diaphragm can undergo consecutive upward and downward movements.
- These movements along with movements of thoracic cage cause rise and fall of the pressure in the thoracic cavity.
- Rising up of ribs and lowering of diaphragm causes the decrease in air pressure which makes the air to move into the lungs through nose. This is inhalation.
- When the pressure rises in the thoracic cavity again, the air is given out. This is exhalation.
- This is caused due to ribs returning to their original position and rising diaphragm. This simultaneously increases the pressure in thoracic cavity.
Question 4.
Structure of human heart:
Answer:
- Human heart is four chambered muscular organ.
- The size of the heart is about one’s own fist and its weight is about 360 gm.
- For protection, it is covered over by double-layered pericardium.
- The wall of the heart is made up of cardiac muscles which are involuntary in nature. They have the capacity of rhythmic beating.
- The upper two chambers are called right and left atrium and lower two chambers are called right and left ventricle.
- Between right atrium and right ventricle there is tricuspid valve which guards the opening. Similarly between left atrium and left ventricle there is bicuspid valve.
- On entire right side of the heart there is deoxygenated blood.
- On entire left side of the heart there is oxygenated blood.
- Right atrium receives deoxygenated blood by superior and inferior vena cava. These two major veins bring deoxygenated blood from entire body to the heart.
- Left atrium receives oxygenated blood from lungs by pulmonary vein.
- Right ventricle sends the deoxygenated blood to lungs for oxygenation, through pulmonary artery.
- Left ventricle supplies oxygenated blood to entire body through systemic aorta.
![]()
Question 5.
Human Blood Groups:
Answer:
- The A, B, O blood group system is the most popular and medically important blood group system of human beings.
- The blood group is determined due to antigenic protein present on the RBCs and the antibody present in the plasma.
- The four main groups of human blood are A, B, AB and O.
- According to the presence or absence of Rh antigen the blood groups are further said to be Rh-positive or Rh-negative.
- This makes total eight blood groups which are taken into consideration at the time of blood transfusions.
- Blood groups are dependent on genes and are thus hereditary in nature.
Answer the following:
Question 1.
How do the organisms like amoeba, earthworm, cockroach, plants, various aquatic animals, bird, respire? Prepare a chart.
Answer:
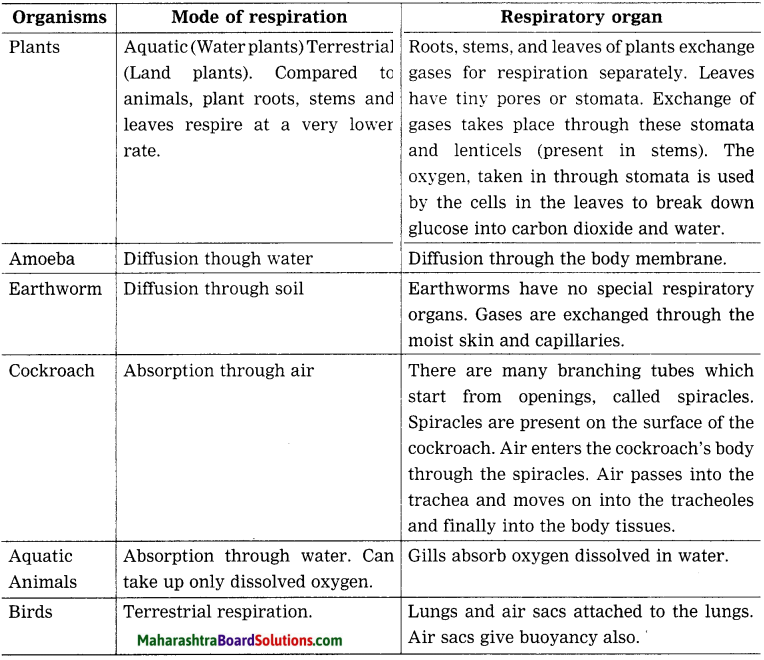
Diagram – based questions:
Question 1.
Sketch and label structure of alveolus showing gaseous exchange.
Answer:
![]()
Question 2.
Sketch and label artery and vein showing structural difference between the two.
Answer:
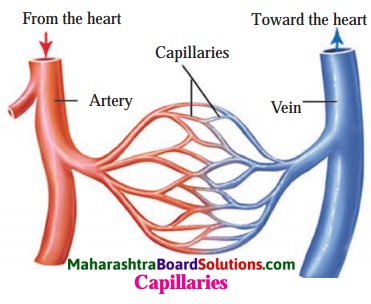
Question 3.
Draw a diagram to show formation of capillaries. Also show how capillaries form a vein.
Answer:
MCQs based on experiments:
I. Human respiratory system:
Question 1.
From which organ does respiratory system of man begin?
(a) Trachea
(b) Lungs
(c) Alveolus
(d) Nose
Answer:
(d) Nose
Question 2.
Which of the statement given below is correct?
(a) Wind pipe is present in front of food pipe.
(b) Food pipe is present in front of wind pipe.
(c) Wind pipe and food pipe are not near each other.
(d) Wind pipe is same as oesophagus and food pipe is called trachea.
Answer:
(a) Wind pipe is present in front of food pipe.
Question 3.
Which type of respiration includes inhalation and exhalation?
(a) Internal respiration
(b) External respiration
(c) Cellular respiration
(d) None of the above
Answer:
(b) External respiration
![]()
Question 4.
How many molecules of ATP are produced from one molecule of glucose ?
(a) 26
(b) 36
(c) 38
(d) 40
Answer:
(c) 38
Question 5.
Where is sound box located in the body?
(a) At the beginning of the wind pipe.
(b) At the beginning of the food pipe.
(c) At the end of the wind pipe.
(d) At the end of the food pipe.
Answer:
(a) At the beginning of the wind pipe.
II. Structure of heart:
Question 1.
What is the location of a bicuspid valve in human heart?
(a) Between right and left ventricle.
(b) Between left and right atrium.
(c) Between left atrium and left ventricle.
(d) Between right atrium and right ventricle.
Answer:
(c) Between left atrium and left ventricle.
Question 2.
Which blood vessels bring deoxygenated blood to the right atrium?
(a) Superior and inferior vena cava
(b) Pulmonary veins
(c) Pulmonary artery
(d) Systemic aorta
Answer:
(a) Superior and inferior vena cava
Question 3.
Which artery carries deoxygenated blood and which vein carries the oxygenated blood respectively?
(a) Dorsal aorta and inferior vena cava respectively.
(b) Pulmonary vein and pulmonary artery respectively
(c) Pulmonary artery and pulmonary vein respectively
(d) Superior vena cava and inferior vena cava respectively.
Answer:
(c) Pulmonary artery and pulmonary vein respectively
Question 4.
Under which of the following condition will rate of heartbeat be faster?
(a) Sleeping
(b) Resting
(c) Running
(d) Sitting
Answer:
(c) Running
![]()
Question 5.
Which chambers of the heart are called receiving chamber?
(a) Both atria
(b) Right ventricle
(c) Left ventricle
(d) Only left atria
Answer:
(a) Both atria