Balbharti Maharashtra State Board 11th Chemistry Important Questions Chapter 14 Basic Principles of Organic Chemistry Important Questions and Answers.
Maharashtra State Board 11th Chemistry Important Questions Chapter 14 Basic Principles of Organic Chemistry
Question 1.
How is the structural formula of a molecule represented? Give an example.
Answer:
Structural formula:
i. Structural formula of a molecule shows all the constituent atoms denoted with their respective chemical symbols and all the covalent bonds therein represented by a dash joining mutually bonded atoms.
ii. Structural formula of methane is:

Question 2.
Write a note on Lewis structures with the help of an example.
Answer:
Lewis structures:
i. The electron dot structures are called as Lewis structures,
e. g. The Lewis structure of methane is shown below.

ii. All the valence electrons of carbon and hydrogen are shown as dots around them. Two dots drawn between two atoms indicate one covalent bond between them. The covalent bond can be represented by a dash joining mutually bonded atoms.
iii. The dash formula represents simplified Lewis formula of the molecule.
e.g. Dash formula of methane:
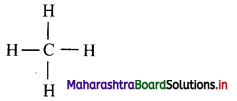
Question 3.
How is the condensed formula of an organic molecule written?
Answer:
The complete structural formula is further simplified by hiding some or all the covalent bonds and indicating the number of identical groups attached to an atom by a subscript. The resulting formula of a compound is known as condensed formula.
e.g.
- The condensed formula of ethane is written as CH3-CH3 or CH3CH3.
- The condensed formula of n-pentane is written as CH3CH2CH2CH2CH3 or CH3(CH2)3CH3.
![]()
Question 4.
What do you understand by the term bond-line formula?
Answer:
Bond-line or zig-zag formula:
i. The condensed formula is simplified into bond-line formula, which is also known as zig-zag formula.
ii. In this representation of an organic molecule, the symbols of carbon and hydrogen atoms are not written. The carbon-carbon bonds are represented by lines drawn in a zig-zag manner
iii. The terminals of the zig-zag line indicate methyl groups and the intersection of lines denote a carbon atom bonded to appropriate number of hydrogen atoms which satisfy the tetravalency of the carbon atom.
e.g. Propane is represented by bond-line or zig-zag formula:
![]()
iv. If a compound contains heteroatom(s) or H-atom(s) bonded to heteroatom(s), then they are represented by their symbols.
e.g. Ethanol is represented by bond-line or zig-zag formula:
![]()
Question 5.
Name the different methods used to represent three-dimensional structure of a molecule on the paper.
Answer:
Four different methods are used to represent three-dimensional structure of a molecule on the paper:
- Wedge formula
- Fischer projection formula or cross formula
- Newman projection formula
- Sawhorse or andiron or perspective formula
Question 6.
Write a short note on: Wedge formula.
Answer:
Wedge formula:
i. The three-dimensional (3-D) structure of organic molecules can be represented on plane paper by using solid![]() and dashed
and dashed ![]() wedges and normal line (-) for single bonds.
wedges and normal line (-) for single bonds.
ii. In this formula, the solid wedge is used to indicate a bond projecting up from the plane of paper, towards the reader (observer), whereas the dashed wedge is used to depict a bond going backward, below the paper away from the reader.
iii. The bonds lying in plane of the paper are depicted by using a normal line (-).
iv. Wedge formula of methane molecule is shown below:

Question 7.
How is Fischer projection formula of a molecule drawn? Explian by giving an example.
Answer:
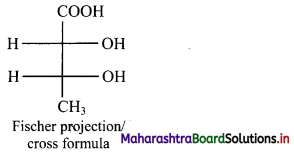
Fischer projection (cross) formula:
- In this representation, a three dimensional molecule is projected on plane of paper.
- Fischer projection formula can be drawn by visualizing the molecule with its main carbon chain vertical.
- Each carbon on the vertical chain is represented by a cross. By convention, the horizontal lines of the cross represent bonds projecting up from the carbon and the vertical lines represent the bonds going below the carbon.
Fischer projection formula of a molecule along with its wedge formula is represented below:
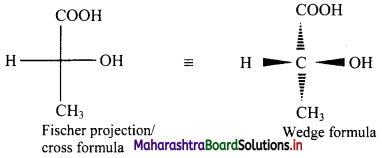
[Note: Fischer projection formula is more commonly used in carbohydrate chemistry.]
![]()
Question 8.
Write the Fischer projection and wedge formula for 2-chloro-propan-2-ol.
Answer:
2-Chloropropan-2-ol has formula CH3C(Cl)(OH)CH3.
Fischer projection and wedge formula for 2-chloropropan-2-ol can be given as:
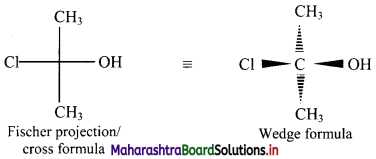
Question 9.
Convert the following wedge formula to Fischer projection formula:
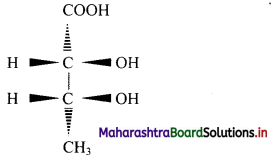
Answer:
Question 10.
Explain how will you represent the Newman projection formula and Sawhorse formula of ethane molecule?
Answer:
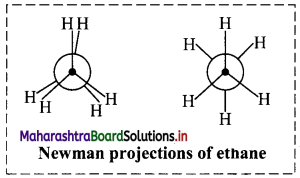
i. Newman projection formula of ethane molecule:
a. A Newman projection views the carbon-carbon single bond directly head-on. The front carbon atom is represented by a point while the rear carbon atom is represented by a circle. The point is drawn at the centre of the circle.
b. Bonds attached to the front carbon atom are represented by three lines drawn at an angle of 120° to each other from the centre of the circle and bonds attached to the rear carbon atom are represented by three lines drawn at an angle of 120° to each other from the circumference of the circle.
c. Newman projections of ethane molecule is represented in adjacent diagram.
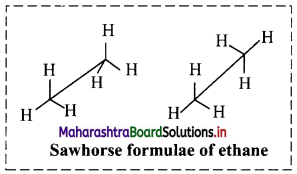
ii. Sawhorse (or andiron or perspective) formula of ethane molecule:
a. In this representation, a C-C single bond is represented by a long slanting line. The lower end of the line represents the front carbon and the upper end represents the rear carbon.
b. The remaining three bonds at the two carbons are shown to radiate from the respective carbons. (As the central C-C bond is drawn rather elongated the bonds radiating from the front and rear carbons do not intermingle.)
c. Sawhorse formula of ethane molecule is represented in adjacent diagram.
Question 11.
Explain the classification of organic compounds based on carbon skeleton.
Answer:
On the basis of their carbon skeleton, organic compounds are classified into two main groups:
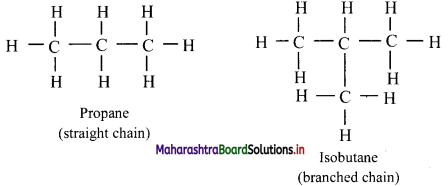
i. Acyclic or aliphatic or open chain compounds:
a. Organic compounds in which carbon atoms are joined to form an open chain are called aliphatic compounds.
b. Their structure may consist of straight chains (in which carbon atoms are bonded to one or two other carbon atoms) or branched chains (in which at least one carbon atom is bonded to three or four other carbon atoms).
e.g.
ii. Cyclic or closed chain or ring compounds:
a. Organic compounds in which carbon atoms are joined to form one or more closed rings with or without hetero atom are called cyclic compounds.
b. They are further divided into two types: Homocyclic and heterocyclic compounds.
1. Homocyclic or carbocyclic compounds: The cyclic organic compounds which have a ring made up of only carbon atoms are called as homocyclic or carbocyclic compounds.
They are further divided into:
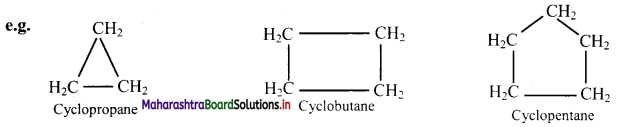
i. Alicyclic compounds: These are cyclic compounds (ring of 3 or more C-atoms) exhibiting properties similar to those of aliphatic compounds.
ii. Aromatic compounds: These compounds have special stability.
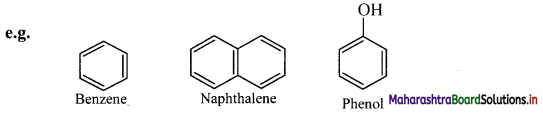
Aromatic compounds are further classified as benzenoid and non-benzenoid aromatics.
a. Benzenoid aromatics contain at least one benzene ring in the structure.
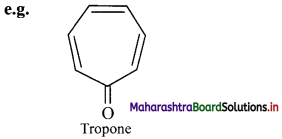
b. Non-benzenoid aromatics contain an aromatic ring, other than benzene.
2. Heterocyclic compounds: Cyclic organic compounds which contain one or more heteroatoms (such as O, N, S, etc.) in the ring are called heterocyclic compounds.
They are further divided into:
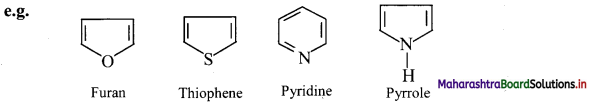
i. Heterocyclic aromatic compounds: Aromatic compounds which contain at least one heteroatom in the ring are called heterocyclic aromatic (hetero-aromatic) compounds.
ii. Heterocyclic non-aromatic compounds: Alicyclic compounds, which contain at least one heteroatom in the ring are called heterocyclic non-aromatic compounds (hetero-alicyclic) compounds.
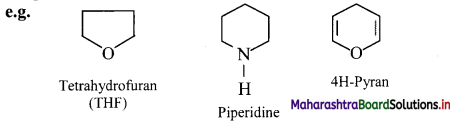
Question 12.
What is a functional group? Give two examples.
Answer:
Functional group:
i. A part of an organic molecule which undergoes change as a result of a reaction is called functional group.
OR
An atom or a group of atoms in the organic molecule which determines its characteristic chemical
properties is called functional group.
e.g. a. The functional group in alcohols is -OH group.
b. The functional group in aldehydes is -CHO group.
ii. There are a large variety of functional groups in organic compounds. Hence, organic compounds can be classified based on the nature of functional group present in them.
iii. The resulting individual class of compounds is called a family and is named after the constituent functional group.
e.g. Family of alcohols, which includes organic compounds having -OH functional group.
Note: Functional groups in organic compounds:
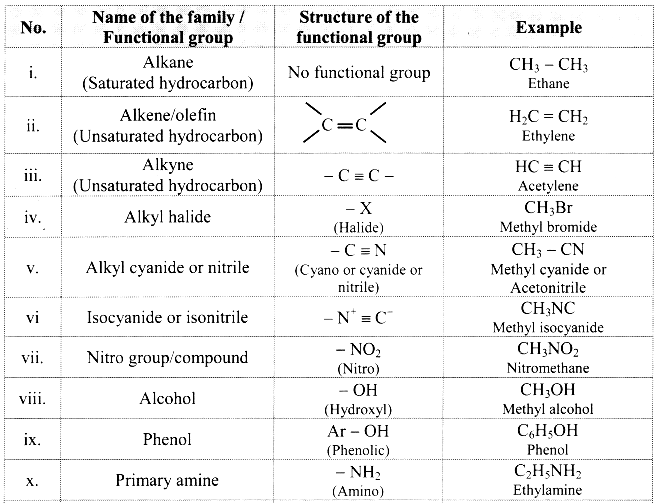
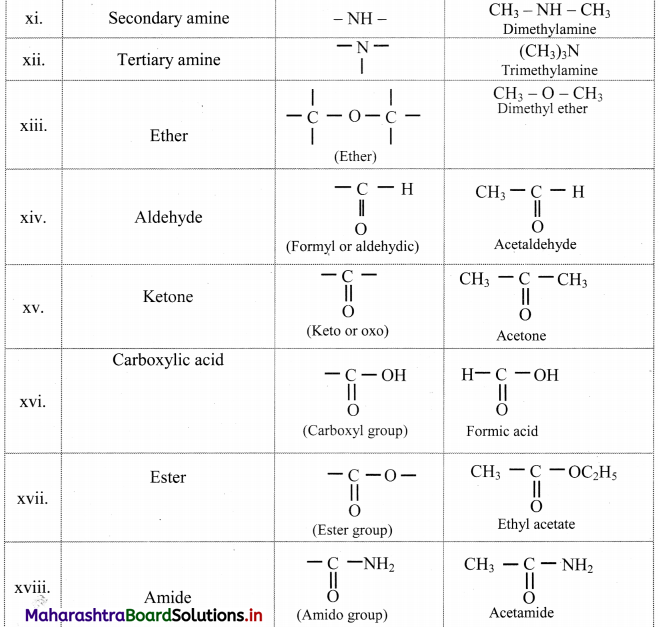
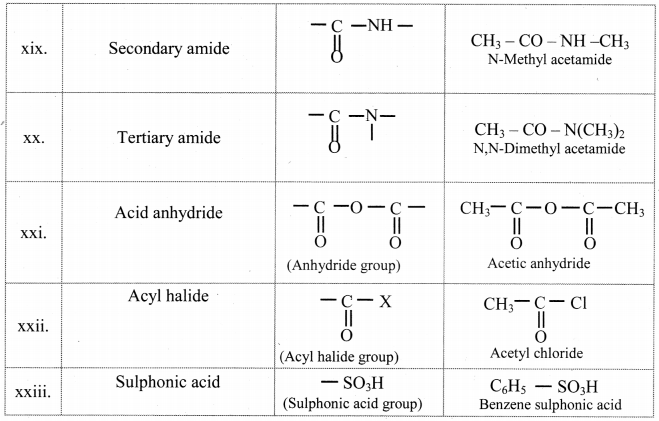
![]()
Question 13.
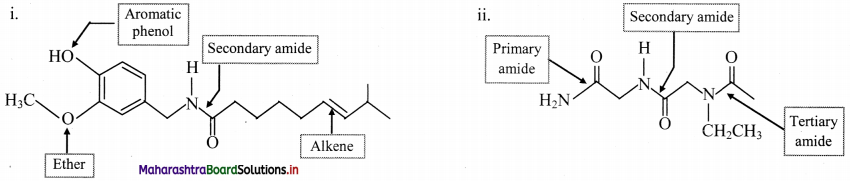
Indicate all the functional groups present in the following compounds.
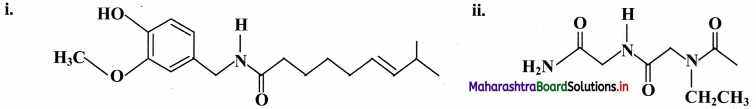
Answer:
Question 14.
Identify the functional group in the following compounds:
i. n-Butyl alcohol
ii. Propanone
iii. Acetylene
Answer:
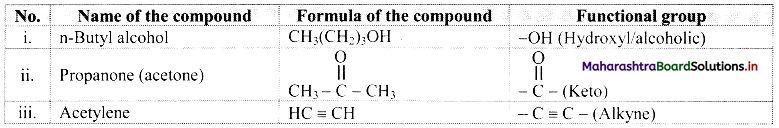
Question 15.
Write the name of the family of the following organic compounds:
i. CH3(CH2)3CH2Cl
ii. CH3CH2CH2NH2
iii. CH3CH2COCH3
iv. CH3CH2OCH3
Answer:

Question 16.
Write a note on homologous series.
Answer:
Homologous series:
- A series of compounds of the same family in which each member has the same type of carbon skeleton and functional group, and differs from the next member by a constant difference of one methylene group (-CH2-) in its molecular and structural formula is called as homologous series.
- The individual members of the series are called homologues and they can be represented by a same general formula.
- Two successive homologues differ by one – CH2 (methylene) unit (i.e., molecular weight of each successive member differs by 14 units).
- Homologues show similar chemical properties.
- Physical properties (like melting point, boiling point, density, solubility, etc.) of the homologues show a gradual change with increase in the molecular weight of the member.
Note: Consider the homologous series of straight chain aldehydes. The boiling point increases down the series as molecular weight increases.
| Name | Molecular formula | Boiling point |
| Formaldehyde | HCHO | -21 °C |
| Acetaldehyde | CH3CHO | 21 °C |
| Propionaldehyde | C2H5CHO | 48 °C |
| Butyraldehyde | C3H7CHO | 75 °C |
| Valeraldehyde | C4H9CHO | 103 °C |
![]()
Question 17.
Alkanes constitute a homologous series of straight chain saturated hydrocarbons. Write down the structural formulae of the first five homologues of this series. Write their molecular formulae and deduce the general formula of such homologous series.
Answer:
The first five homologues are generated by adding one – CH2 – at a time, starting with the first homologue, methane (CH4).
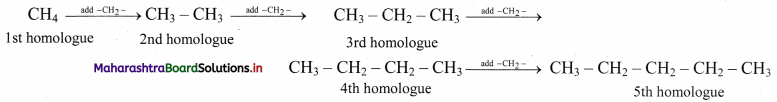
By counting carbon and hydrogen atoms in the five homologues, we get their molecular formulae as CH4, C2H6, C3H8, C4H10 and C5H12.
Comparing these molecular formulae and assigning the number of carbon atoms as ‘n’, the following general formula is deduced: CnH2n+2.
Question 18.
Write down structural formulae of (i) the third higher and (ii) the second lower homologue of CH3CH2COOH.
Answer:
i. Structural formula of the third higher homologue is obtained by adding three – CH2 – units to the carbon chain of the given structure.
![]()
ii. Structural formula of the second lower homologue is obtained by removing two – CH2 – units from the carbon chain of the given structure.
![]()
Question 19.
Write the general formula of homologous series of alcohols.
Answer:
General formula of homologous series of alcohols can be represented as, CnH2n+1OH (where n = 1, 2, 3, …).
Question 20.
Write the name and molecular formulae of the first three higher homologues of propyl chloride.
Answer:
General formula: CnH2n+1Cl (where n = 1, 2, 3, …)
| No. of carbon atoms | Molecular formula | Name |
| n = 3 | C3H7Cl | Propyl chloride |
| n = 4 | C4H9Cl | Butyl chloride |
| n = 5 | C5H11Cl | Pentyl chloride |
| n = 6 | C6H13Cl | Hexyl chloride |
Question 21.
What is the molecular formula of:
i. first higher homologue of propionic acid?
ii. first lower homologue of propionic acid?
Answer:
i. First higher homologue of propionic acid:
(Addition of 1-CH3 group to CH3CH2COOH)
Butyric acid: C3H7COOH
ii. First lower homologue of propionic acid:
(1-CH3 group less from CH3CH3COOH)
Acetic acid: CH3COOH
![]()
Question 22.
How are the saturated (sp3) carbon atoms in a molecule classified based on the number of other carbon atoms bonded to it? Give an example that has all the four types of carbon atoms.
Answer:
i. The saturated (sp3) carbons in a molecule are classified as primary, secondary, tertiary and quaternary in accordance with the number of other carbons bonded to it by single bonds.
- Primary carbon atom (1°): This carbon atom is bonded to only one other carbon atom. Terminal carbon atoms are always 1° carbon atoms.
- Secondary carbon atom (2°): This carbon atom is bonded to two other carbon atoms.
- Tertiary carbon atom (3°): This carbon atom is bonded to three other carbon atoms.
- Quaternary carbon atom (4°): This carbon atom is bonded to four other carbon atoms.
ii. An example molecule having all the four types of carbon atoms:
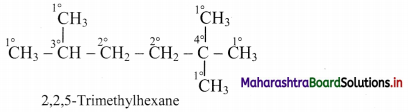
Thus, in 2,2,5-trimethylhexane, there are five primary, two secondary, one tertiary and one quaternary carbon atoms.
[Note: Hydrogen atoms attached to primary’, secondary and tertiary carbon atoms are referred to as primary, secondary and tertiary H-atoms respectively.]
Question 23.
Give common name/trivial name of the following compounds.

Answer:
i. Lactic acid
ii. Glycine
iii. Glycerol
iv. Chloroform
Question 24.
Give a basic idea about IUPAC nomenclature system and comment on IUPAC names of straight chain alkanes.
Answer:
i. International Union of Pure and Applied Chemistry (IUPAC) was founded (in 1919) and a systematic method of nomenclature for organic compounds was developed under its banner.
ii. This was done because of growing number of organic compounds with increasingly complicated structures and it was difficult to name them. To simplify and avoid confusions, IUPAC system is accepted and widely used all over the world today. According to this system, a unique name is given to each organic compound.
Following things are taken into consideration while naming a particular organic compound:
- To arrive at the IUPAC name of an organic compound, its structure is considered to be made of three main parts: parent hydrocarbon, branches and functional groups.
- The IUPAC names of a compound are obtained by modifying the name of its parent hydrocarbon further incorporating names of the branches and functional groups as prefix and suffix.
IUPAC names of straight chain alkanes:
a. The homologous series of straight chain alkanes forms the parent hydrocarbon part of the IUPAC names of aliphatic compounds.
b. The IUPAC name of a straight chain alkane is derived from the number of carbon atoms it contains.
c. IUPAC names of the first twenty alkanes are mentioned in the following table:
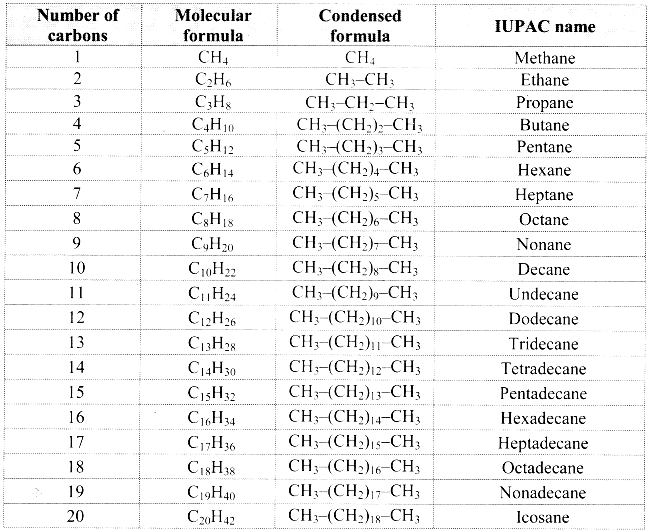
![]()
Question 25.
Match the following:
| Column – I | Column – II | ||
| i. | C19H40 | a. | Undecane |
| ii. | C12H26 | b. | Nonadecane |
| iii. | C11H24 | c. | Dodecane |
| d. | Nonane |
Answer:
i – b,
ii – c,
iii – a
Question 26.
Explain the following with two examples:
i. straight chain alkyl groups
ii. branched chain alkyl group
Answer:
i. Straight chain alkyl group: It is obtained by removing one H-atom from the terminal carbon of an alkane molecule.
ii. It is named by replacing ‘ane’ of the alkane by ‘yl’.
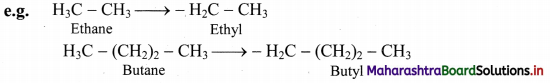
iii. Branched chain alkyl group: It is obtained by removing a H-atom from any one of the non-terminal carbons of an alkane or any H-atom from a branched alkane.
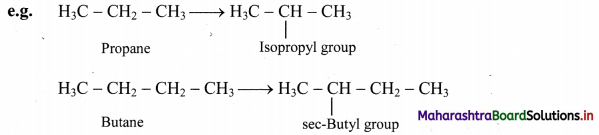
Note: Straight chain alkyl groups
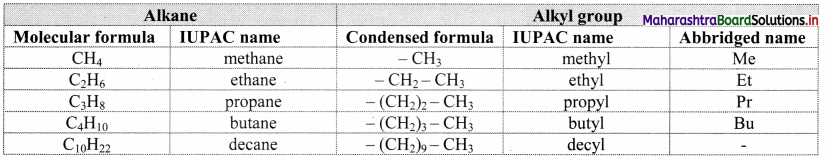
Trivial names of small branched alkyl groups
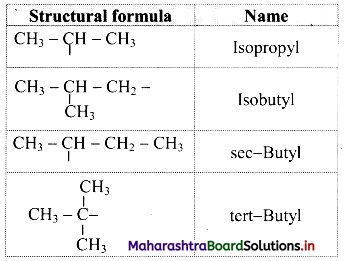
Question 27.
Write names of following groups.
i. C6H5–
ii. (CH3)3C-
Answer:
i. Phenyl group
ii. tert-Butyl group
Question 28.
State the rules to assign IUPAC nomenclature of a branched chain alkane.
Answer:
i. Select the longest continuous chain of carbon atoms to be called the parent chain. All other carbon atoms not included in this chain constitute, side chains or branches or alkyl substituents. For example:
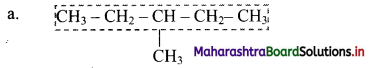
Parent chain has five carbon atoms and -CH3 group is alkyl substituent.
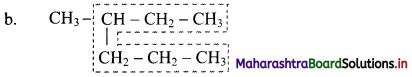
Parent chain has six carbon atoms and methyl group is the alkyl substituent.
If two chains of equal length are located, then the one with maximum number of substituents is selected as the parent chain.
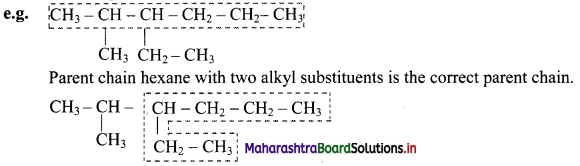
Parent chain hexane with one alkyl substituent is the incorrect chain.
ii. The parent chain is numbered from one end to the other to locate the position, called locant number of the alkyl substituent. The numbering is done in that direction which will result in lowest possible locant numbers.

iii. Names of the alkyl substituents are added as prefix to the name of the parent alkane. Different alkyl substituents are listed in alphabetical order with each substituent name preceded by the appropriate locant number. The name of the substituent is separated from the locant number by a hyphen.
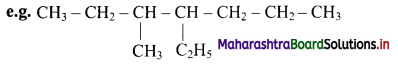
The name is 4-ethyl-3-methylheptane and not 3-methyl-4-ethylheptane.
iv. When both the numberings give the same set of locants, that numbering is chosen which gives smaller locant to the substituent having alphabetical priority.
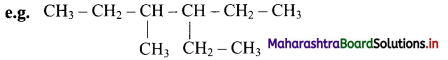
The name is 3-ethyl-4-methylhexane and not 3-methyl-4-ethylhexane.
v. If two or more identical substituents are present the prefix di (for 2), tri (for 3), tetra (for 4) and so on, are used before the name of the substituent to indicate how many identical substituents are there. The locants of identical substituents are listed together, separated by commas.
There must be as many numbers in the name as the substituents. A digit and an alphabet are separated by hyphen. The prefixes di, tri, tetra, sec and tert are ignored in alphabetizing the substituent names. Substituent and parent hydrocarbon names are joined into one word.
vi. Branched alkyl group having no accepted trivial name is named with the longest continuous chain beginning at the point of attachment as the base name. Carbon atom of this group attached to parent chain is numbered as ‘1’. The name of such substituent is enclosed in bracket.
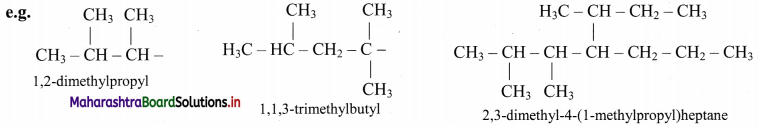
Question 29.
Complete the following table.
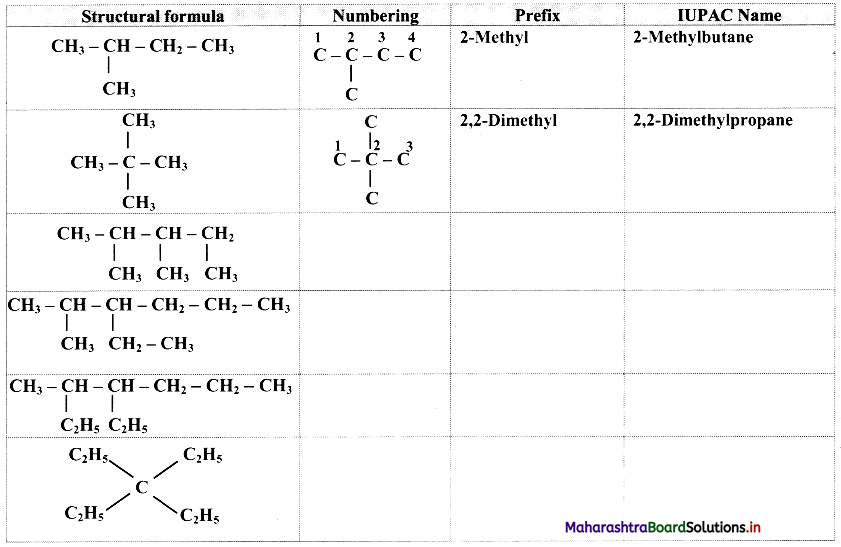
Answer:
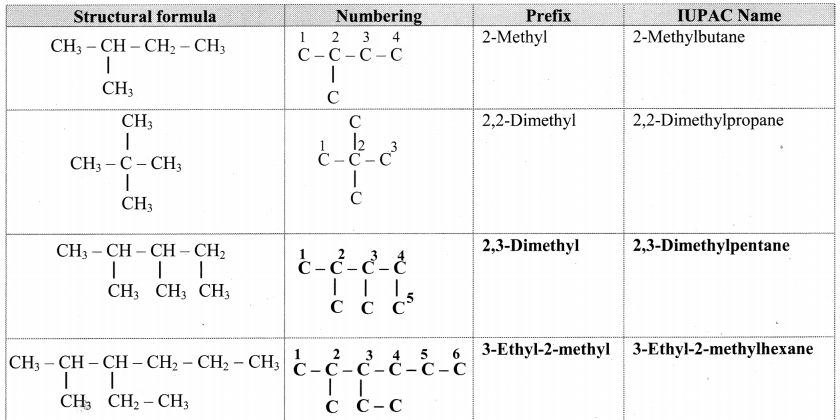
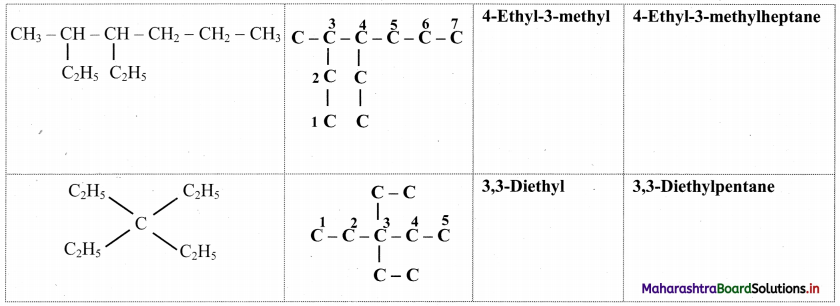
![]()
Question 30.
Explain the rules for IUPAC nomenclature of unsaturated hydrocarbons (Alkenes and Alkynes).
Answer:
While writing IUPAC names of alkenes and alkynes following rules are to be followed in addition to rules for alkanes.
i. The longest continuous chain must include carbon-carbon multiple bond. Thus, the longest continuous chains in 1 and II contain four and six carbons, respectively.

ii. Numbering of this chain must be done such that carbon-carbon multiple bond has the lowest possible locant number.

iii. The ending ‘ane’ of alkane is replaced by ‘ene’ for an alkene and ‘yne’ for an alkyne.
iv. Position of carbon atom from which multiple bond starts is indicated by smaller locant number of two multiple bonded carbons before the ending ‘ene’ or ‘yne’. e.g.

v. If the multiple bond is equidistant from both the ends of a selected chain, then carbon atoms are numbered from that end, which is nearer to first branching.

vi. If the parent chain contains two double bonds or two triple bonds, then it is named as diene or diyne. In all these cases ‘a’ of ‘ane’ (alkane) is retained.
![]()
vii. If the parent chain contains both double and triple bond, then carbon atoms are numbered from that end where multiple bond is nearer. Such systems are named by putting ‘en’ ending first followed by ‘yne’. The number indicating the location of multiple bond is placed before the name.
![]()
viii. If there is a tie between a double bond and a triple bond, the double bond gets the lower number.
![]()
Question 31.
Give IUPAC rules for naming simple monocyclic hydrocarbons.
Answer:
i. A saturated monocyclic hydrocarbon is named by attaching prefix ‘cyclo’ to the name of the corresponding open chain alkane.
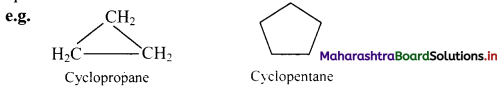
ii. An unsaturated monocyclic hydrocarbon is named by substituting ‘ene’, ‘yne’, etc. for ‘ane’ in the name of corresponding cycloalkane.
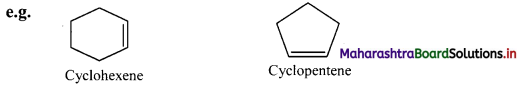
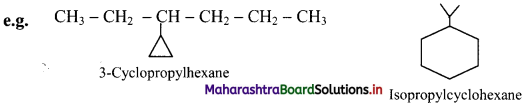
iii. If side chains are present then the numbering of the ring carbon is started from a side chain.

iv. If alkyl groups contain greater number of carbon atoms than the ring, the compound is named as derivative of alkane. Ring is treated as substituent.
Question 32.
Give the IUPAC names of the following compounds:

Answer:
i. 1-Ethyl-1-methyl-2-propylcyclohexane
ii. 1,2-Dimethylcyclobutane
iii. Cyclopentene
iv. 3-Cyclopropylhex-1-yne
Question 33.
Explain in short how naming of monofunctional compound is done.
Answer:
Naming of monofunctional compounds: When a molecule contains only one functional group, the longest carbon chain containing that functional group is identified as the parent chain and numbered so as to give the smallest locant number to the carbon bearing the functional group. The parent name is modified by applying appropriate suffix. Location of the functional group is indicated where necessary and when it is NOT numbered ‘1’.
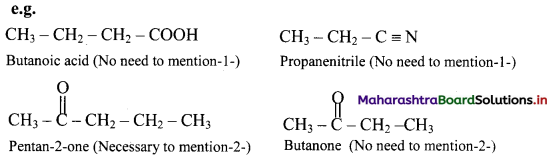
When the functional group cannot be used as suffix, and can be only the prefix, the molecule is named as parent alkane carrying the functional group as substituent at specified carbon.

Question 34.
Complete the following.
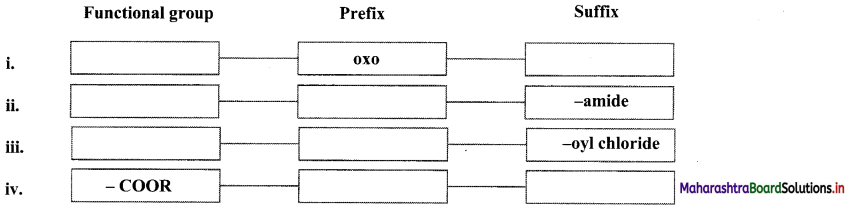
Answer:
Question 35.
Give examples of functional groups which can appear only as prefix?
Answer:
Functional groups which can appear only as prefix are as follows:
i. Nitro group (-NO2)
ii. Halides (-X): Represented by prefix “halo” (like fluoro, chloro, bromo, iodo).
iii. Alkoxy group (-OR): Groups like methoxy (-OCH3), ethoxy (-OC2H5), etc.
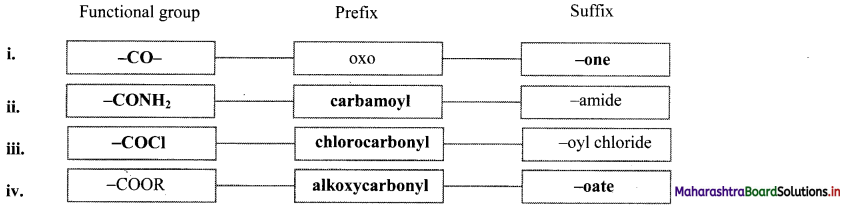
Note: Functional groups appearing as prefix and suffix
| Functional Group | Prefix | Suffix |
| -COOH | Carboxy | – oic acid |
| -COOR | alkoxycarbonyl | – oate |
| -COCl | Chlorocarbonyl | – oyl chloride |
| -CONH2 | Carbamoyl | – amide |
| -CN | Cyano | – nitrile |
| -CHO | Formyl | – al |
| -CO- | Oxo | – one |
| -OH | Hydroxy | – ol |
| -NH2 | Amino | – amine |
![]()
Question 36.
Write a note on principal functional group.
Answer:
i. The organic compounds possessing two or more functional groups (same or different) in their molecules are called polyfunctional compounds.
ii. When there are two or more different functional groups, one of them is selected as the principal functional group and the others are considered as substituents.
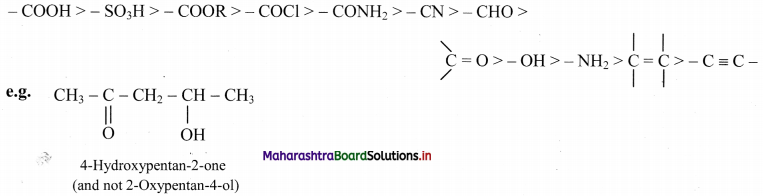
iii. The principal functional group is used as suffix of the IUPAC name while the other substituents are written with appropriate prefixes. The principal functional group is decided on the basis of the following order of priority:
Question 37.
Explain the rules for naming mono or polyfunctional compounds.
Answer:
- Identification of parent chain: The longest carbon chain containing the single or the principal functional group is identified as parent chain.
e.g. Ethers are named as alkoxyalkane. While naming it, the larger alkyl group is chosen as parent chain. - Numbering of parent chain: It is done so as to give the lowest possible locant numbers to the carbon atom of this functional group.
- Suffix: The name of the parent hydrocarbon is modified adequately with appropriate suffix in accordance with the single/principal functional group.
- Names of the other functional groups (if any) are attached to this modified name as prefixes. The locant numbers of all the functional groups are indicated before the corresponding suffix/prefix.
[Note: The carbon atom in -COOR, -COCl, -CONH2, -CN and -CHO is C – 1 by rule and therefore, is not mentioned in the IUPAC name.]
Question 38.
Write IUPAC names for the following structures:

Answer:
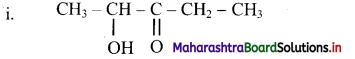
Here, the principal functional group, ketone is located at the C-3 on the five carbon chain. The -OH group, the hydroxyl substituent is at C-2. Therefore, the IUPAC name is 2-hydroxypentan-3-one.
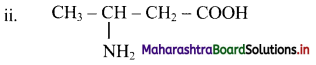
Here, the principal functional group is carboxylic acid. The amino substituent is located at C-3 on four carbon chain. Therefore, the IUPAC name 3-aminobutanoic acid.
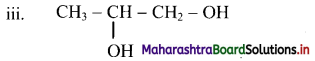
Here, two same functional groups are present at C-1 and C-2 position. They are indicated by using the term ‘di’ before the class suffix. Therefore, the IUPAC name is propane-1,2-diol.
iv. CH2 = CH – CH = CH2
Here, the parent chain contains two double bonds at C-1 and C-3, hence it is named as diene. Therefore, the IUPAC name is buta-1,3-diene.
Question 39.
Give IUPAC rules for naming substituted benzene.
Answer:
i. Monosubstituted benzene : The IUPAC name of a monosubstituted benzene is obtained by placing the name of substituent as prefix to the parent skeleton which is benzene.
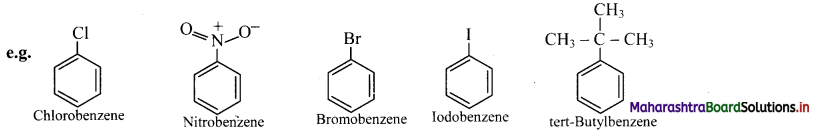
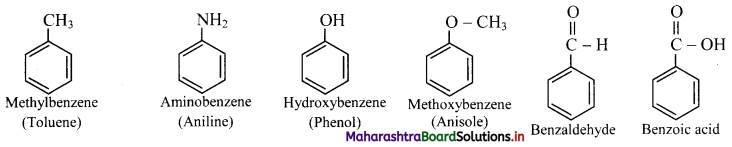
ii. Some monosubstituted benzenes have trivial names which may show no resemblance with the name of the attached substituent group. For example, methylbenzene is known as toluene, aminobenzene as aniline, hydroxybenzene as phenol and so on. The common names written in the bracket are also used universally and accepted by IUPAC.
iii. If the alkyl substituent is larger than benzene ring (7 or more carbon atoms) the compound is named as phenyl-substituted alkane.

iv. Benzene ring can as well be considered as substituent when it is attached to an alkane with a functional group.
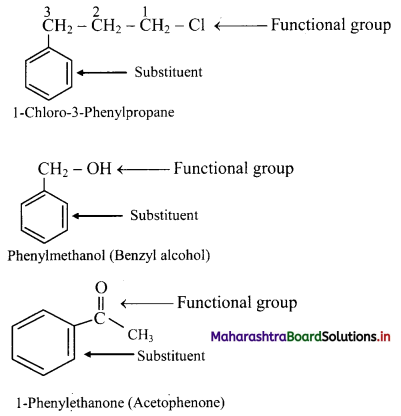
v. Disubstituted benzene derivatives:
Common names of the three possible isomers of disubstitued benzene derivatives are given using one of the prefixes ortho (o-), meta (m-) or para (p-).
IUPAC system, however, uses numbering instead of prefixes, o-, m-, or p-.
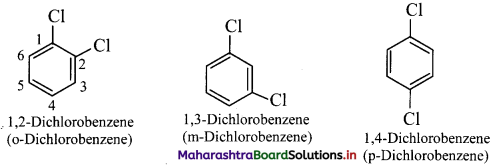
vi. If two substituents are different, then they enter in alphabetical order.
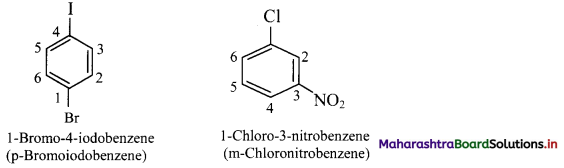
vii. If one of the two groups gives special name to the molecule then the compound is named as derivative of the special compound.
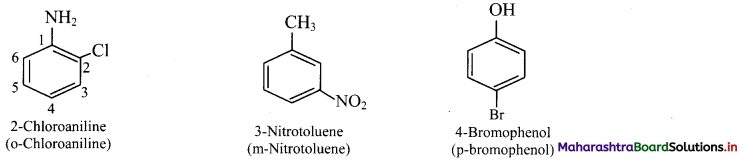
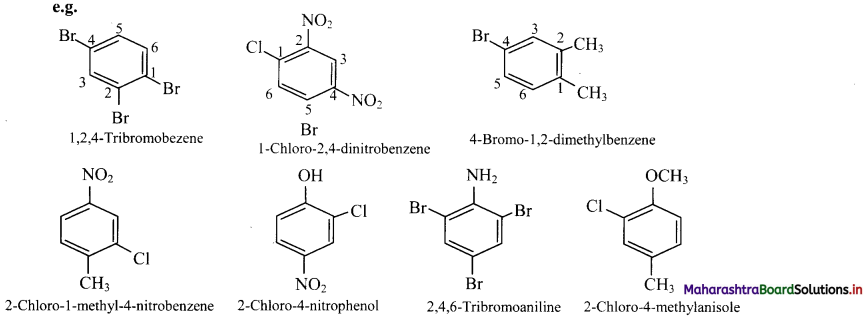
viii. Trisubstituted benzene derivatives : If more than two substituents are attached to benzene ring, numbers are used to indicate their relative positions following the alphabetical order and lowest locant rule. In some cases, common name of benzene derivatives is taken as parent compound.
Question 40.
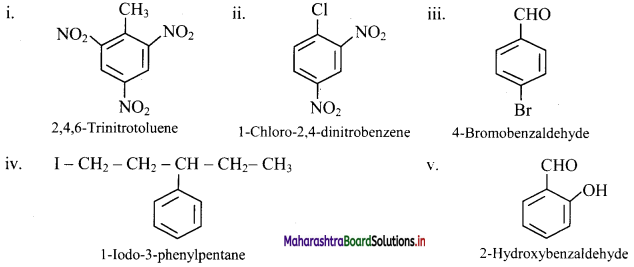
Write the structural formula of following derivatives of benzene.
i. 2,4,6-Trinitrotoluene
ii. 1-Chloro-2,4-dinitrobenzene
iii. 4-Broniobenzaldehyde
iv. 1-Iodo-3-phenylpentane
v. 2-Hydroxybenzaldehyde
Answer:
Question 41.
Write the IUPAC names of the following compounds.

Answer:
i. 5-Phenylpent-1-ene
ii. 2-Hydroxybenzoic acid
![]()
Question 42.
Define the terms:
i. Isomerism
ii. Isomers
Answer:
i. Isomerism: The phenomenon of existence of two or more compounds possessing the same molecular formula is known as isomerism.
ii. Isomers: Two or more compounds having the same molecular formula are called as isomers of each other. [Note: The isomers are different compounds having same molecular formula and therefore they exhibit different physical and chemical properties.]
Question 43.
Define: Structural isomerism
Answer:
Structural isomerism: When two or more compounds have same molecular formula but different structural formulae, they are said to be structural isomers of each other and the phenomenon is known as structural isomerism.
Question 44.
Define: Stereoisomerism
Answer:
When different compounds have the same structural formula but different relative arrangement of groups/atoms in space, that is, different spatial arrangement of groups/atoms, it is called as stereoisomerism.
Question 45.
Give different types of structural isomerism that organic compounds can exhibit.
Answer:
Different types of structural isomerism that organic compounds may exhibit are as follows:
- Chain isomerism
- Position isomerism
- Functional group isomerism
- Metamerism
- Tautomerism
Question 46.
Explain chain isomerism in alkanes with two suitable examples.
Answer:
Chain isomerism: When two or more compounds have the same molecular formula but different parent chain or different carbon skeletons, it is referred to as chain isomerism and such isomers are known as chain isomers.
e.g.
i. Butane (C4H10) exists in two isomeric forms:

Here, n-butane contains longest chain of four carbon atoms whereas isobutane contains longest chain of three carbon atoms. Such isomers having different carbon skeletons are called as chain isomers.
[Note: Methylpropane has no other branched isomers, hence locant (2) can be dropped.]
ii. Pentene (C5H12) exists in three isomeric forms:
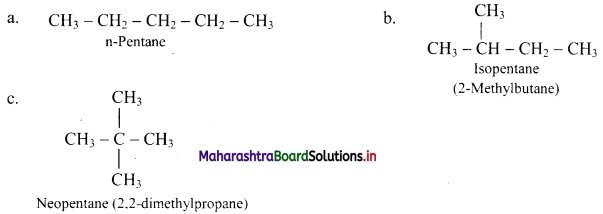
[Note: The numbers of chain isomers increase with the increase in the number of carbon atoms in the molecule.]
![]()
Question 47.
Write a note on position isomerism.
Answer:
i. The phenomenon in which diffèrent compounds having the same functional group at different positions on the parent chain is known as position isomerism.
ii. e.g. But-1-ene and but-2-ene are position isomers of each other as they have the same molecular formula (C4H8) and the sanie carbon skeleton hut the double bonds are located at different positions.

Question 48.
Define: Functional group isomerism
Answer:
Different compounds having the same molecular formula but different functional groups are called as futictional group isomers and the phenomenon is called as junctional group isomerism.
e.g. CH3 – O – CH3 (Dimethyl ether) and C2H5 – OH (ethyl alcohol) have same molecular formula (C2H6O) but former has ether (-O-) functional group and the latter has alcoholic (-OH) functional group.
Question 49.
Explain: Metamerism
Answer:
i. Metamerism may be defined as a type of isomerism in which different compounds have same molecular formula and the same functional group but have unequal distribution of carbon atoms on either side of the functional group. Such isomers are known as metamers.
ii. e.g. Ether with molecular formula C4H10O has three metamers. They have same functional group as ether but have different distribution of carbon atoms attached to etheral oxygen. These metamers are:

Question 50.
Explain: Tautomerism
Answer:
When same compound exists as mixture of two or more structurally distinct molecules which are in rapid equilibrium with each other, then the phenomenon is referred to as tautomerism. Such interconverting isomers are called tautomers.
i. In nearly all the cases, it is the proton which shifts from one atom to another atom in the molecule to form its tautomer.
ii. Keto-enol tautomerism is very common form of tautomerism.
iii. Here, a hydrogen atom shifts reversibly from the a-carbon of the keto form to oxygen atom of the enol. This type of isomerism is known as keto-enol tautomerism.
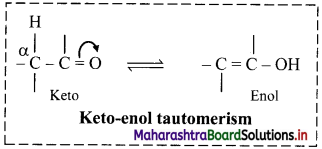
Question 51.
Explain the terms substrate, reagent and byproduct in an organic reaction.
Answer:
- Organic molecules primarily contain various types of covalent bonds between the constituent atoms. During an organic reaction, molecules of the reactant undergo change in their structure. A covalent bond at a carbon atom in the reactant is broken and a new covalent bond is formed at it, giving rise to the product.
- The reactant that provides carbon to the new bond is called substrate. In other words, substrate is a chemical species which reacts with reagent to give corresponding products.
- The other reactant which brings about this change is called reagent.
- Apart from the product of interest, some other products are also formed in an organic reaction. These are called byproducts.
e.g. In following reaction, methane is the substrate and chlorine is the reagent. The product of interest is methyl chloride and the byproduct is HCl.
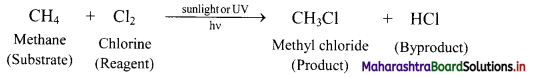
Question 52.
Explain: Organic reactions are often a multi-step process.
Answer:
- Organic molecules contain covalent bonds, which are made of valence electrons of the constituent atoms.
- During an organic reaction, molecules of the reactant undergo change in their structure due to redistribution of valence electrons of constituent atoms.
- This results in the bond breaking or bond forming processes as organic reaction proceeds. However, these processes are usually not instantaneous.
- As a result of this, the overall organic reaction occurs by the formation of one or more unstable species called intermediates.
Thus, organic reactions are often a multi-step process.
![]()
Question 53.
What do you mean by reaction mechanism? Give importance of reaction mechanism.
Answer:
i. Mechanism of an organic reaction is the complete step by step description of exactly which bonds break and which bonds form, in what manner and in what order to give the observed products.
ii. In general, reaction mechanism is a sequential account of:
- the electron movement taking place during each step
- the bond cleavage and/or bond formation
- accompanying changes in energy and shapes of various species and
- rate of the overall reaction.
The individual steps, constitute the reaction mechanism.
iii. Importance of reaction mechanism:
The knowledge of mechanism of a reaction is useful for understanding the reactivity of the concerned organic compounds and, in turn, helpful for planning synthetic strategies.
Question 54.
What are the different ways in which a covalent bond fission can takes place?
Answer:
The covalent bond fission/cleavage takes place in two ways:
- Homolytic fission
- Heterolytic fission
Question 55.
Explain homolytic cleavage of a bond with suitable example.
Answer:
Homolytic cleavage:
i. A covalent bond consists of two electrons (i.e., a bond pair of electrons) shared between the two bonded atoms.
ii. In homolytic cleavage of a covalent bond, one of the two electrons go to one of the bonded atoms and the other is bound to the other atom.
iii. This type of cleavage gives rise to two neutral species carrying one unpaired electron each. Such a species with single unpaired electron is called as free radical.
iv. The free radicals are short lived (transitory) and unstable. Therefore, they are very reactive, having tendency to seek an electron for pairing.
v. Homolytic cleavage can be represented as follows:
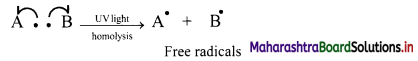
where movement of a single electron is represented by a half-headed curved arrow or fish hook,
vi. Thus, the symmetrical breaking of a covalent bond between two atoms such that each atom retains one electron of the shared pair forming free radicals is known as homolytic cleavage (homolysis).
Question 56.
What conditions favour homolytic cleavage?
Answer:
Homolytic cleavage is favoured in the presence of UV radiation or in presence of catalyst such as peroxides (H2O2) or at high temperatures.
Question 57.
Write a short note on free radical.
Answer:
Free radical:
i. A species with unpaired electron is called free radical.
OR
An uncharged species which is electrically neutral and which contains a single electron is called free radical.
ii. A free radical is highly reactive, unstable and therefore has a transitory existence (short-lived).
iii. Free radicals are formed as reaction intermediate which subsequently react with another radical/molecule to restore stable bonding pair.
iv. In a carbon free radical, the carbon atom having unpaired electron is sp hybridized and has planar trigonal geometry.
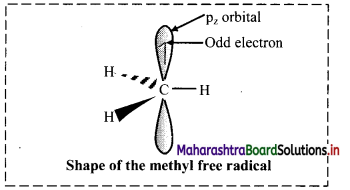
v. The alkyl free radicals are classified as primary, secondary or tertiary depending upon the number of carbon atoms attached to the C-atom carrying the unpaired electron.
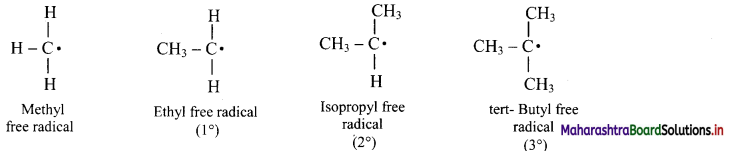
vi. Stability of alkyl free radicals decreases in the order 3° > 2° > 1° > methyl free radical.
![]()
Question 58.
Explain heterolytic cleavage with suitable example.
Answer:
Heterolytic cleavage:
i. In hetcrolytic cleavage of a covalent bond, both shared electrons go to one of the two bonded atoms.
ii. This type of cleavage gives rise to two charged species, one with negative charge (anion) and the other with positive charge (cation).
iii. The negatively charged species has the more electronegative atom which has taken away the shared pair of electrons with it.
iv. Heterolytic cleavage can be represented as follows:
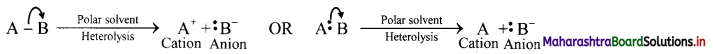
Where B is more electronegative than A and the movement of an electron pair is represented by a curved arrow.
v. Thus, the unsymmetrical breaking of a covalent bond between two atoms in such a way that the more electronegative atom acquires both the electrons of the shared pair. thereby fòrming charged ions is known as heterolytic fission or heterolysis.
Question 59.
What is carbocation? Explain with the help of an example and comment on the stability of carbocation.
Answer:
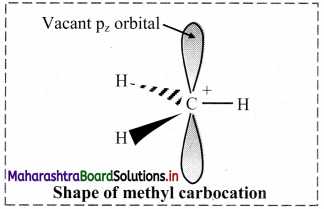
Carbocation:
i. A carbon atom having sextet of electrons and a positive charge is called a carbocation.
ii. They are unstable and highly reactive species formed as intermediates in many organic reactions.
iii. In a carbocation, the central carbon atom is sp2 hybridized and has trigonal planar geometry.
e. g. In a methyl carbocation C If, the positively charged carbon atom is covalently bonded to three hydrogen atoms. It is planar with H-C-H bond angle of 120°.
The unhybridized pz orbital is vacant and lies perpendicular to the plane containing the three sigma C-H bonds.
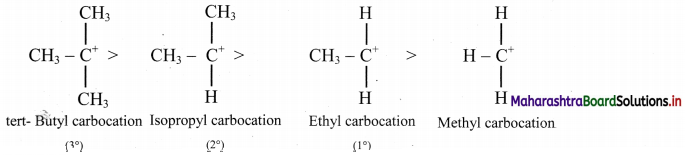
iv. Carbocation are classified as primary (1°), secondary (2°) and tertiary (3°).
v. The stability of carbocations decreases in the order:
Question 60.
Write a short note on carbanion.
Answer:
Carbanion:
i. Carbanion is a species with a negatively charged carbon atom having complete octet (eight electrons) in its valence shell.
ii. It is formed due to heterolytic bond fission when carbon atom is bonded to the more electropositive atom.

(Where Z is more electropositive than C)
iii. Carbanions are unstable and highly reactive species formed as intermediates in many organic reactions.
Question 61.
Give the types of reagents used to carry out polar organic reactions.
Answer:
The polar organic reactions are brought about by two types of reagents.
Depending upon the ability to accept or donate electrons from or to the substrate, reagents are classified as
- Electrophiles (E+)
- Nucleophiles (Nu:)
![]()
Question 62.
Explain the term electrophile. Give examples.
Answer:
Electrophiles:
i. The species which accept electron pairs from the substrate during the reaction are called electrophiles.
ii. The electrophiles are electron seeking (or electron loving) species because they themselves are electron deficient.
iii. e.g. a. Positively charged/cationic electrophiles:
![]()
b. Neutral species with vacant orbitals or incomplete octet of electrons in the outermost orbit: AlCl3, BF3, FeCl3, SO2, BeCl2, ZnCl2, PCl5, etc.
iv. A polyatomic electrophile has an electron deficient atom in it called the electrophilic centre.
e.g. The electrophilic centre of the electrophile AlCl3 is AlCl3 which has only 6 valence electrons.
Question 63.
Explain the term nucleophile. Give examples.
Answer:
Nucleophiles:
i. The species which donate (give away) electron pairs to the substrate during the reaction are called nucleophiles.
ii. Since, nucleophiles are electron rich species, they donate a pair of electrons to acceptor atoms and thus, they are nucleus seeking (or nucleus loving) species.
iii. e.g. a. Negatively charged nucleophiles: OH–, CN–, Cl–, Br–, etc.
b. Neutral species containing at least one lone pair of electrons:
H2O, NH3, H2S, R – OH, R – NH2, R – OR, etc.
iv. A polyatomic nucleophile has an electron rich atom in it called the nucleophilic centre.
e.g. The nucleophilic centre of the nucleophile H2O is ‘O’ which has two lone pairs of electrons.
Question 64.
Identify the nucleophile and electrophile from NH3 and \(\stackrel{+}{\mathrm{C}} \mathrm{H}_{3}\). Also indicate the nucleophilic and electrophilic centres in them. Justify.
Answer:
The structural formulae of two reagents showing all the valence electrons are:
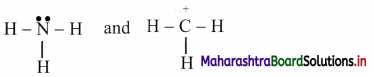
Thus, NH3 contains N with a lone pair of electrons which can be given away to another species. Therefore, NH3 is a nucleophile and ‘N’ in it is the nucleophilic centre.
The \(\stackrel{+}{\mathrm{C}} \mathrm{H}_{3}\) is a positively charged electron deficient species having a vacant orbital on the carbon. It is an electrophile and the ‘C’ in it is the electrophilic centre.
Question 65.
What is the difference between nucleophilic reaction and electrophilic reaction. Give one example.
Answer:
In nucleophilic reaction nucleophile attacks the electrophilic centre in the substrate and brings about a nucleophilic reaction whereas, in electrophilic reaction an electrophile attacks a nucleophilic centre in the substrate and brings about an electrophilic reaction.
![]()
Here, the nucleophilic centre N: in the nucleophile NH3 attacks the electrophilic centre ‘B’ in the electrophile BF3 to form the product.
[Note: Given reaction is not an organic reaction.]
![]()
Question 66.
How electrophilic or nucleophilic centre is generated in a neutral substrate?
Answer:
- The displacement of valence electrons resulting in polarization of an organic molecule is called electronic effect.
- Polarization can be either due to the presence of an atom or substituent group, or due to the influence of certain atornattacking reagent or due to the certain structural feature present in the molecule.
- Such polarization results in the formation of electrophilic or nucleophilic centre in the neutral organic molecule.
Question 67.
Explain the difference between permanent electronic effect and temporary electronic effect.
Answer:
i. Permanent electronic effect:
The electronic effect that occurs in a substrate in the ground state is a permanent effect.
e.g. Inductive effect and resonance effect are two examples of permanent electronic effect.
ii. Temporary electronic effect:
The electronic effect that occurs in a substrate due to approach of the attacking reagent is a temporary effect. This type of electronic effect is called as electromeric effect or polarizability effect.
Question 68.
Define: Inductive effect
Answer:
Inductive effect: When an organic molecule has a polar covalent bond in its structure, polarity is induced in adjacent carbon-carbon single bonds too. This effect is called as inductive effect.
Question 69.
Describe inductive effect in detail.
Answer:
Inductive effect:
i. When an organic molecule has a polar covalent bond in its structure, polarity is induced in adjacent carbon- carbon single bonds too. This effect is called as inductive effect.
ii. For example, in chloroethane molecule, the covalent bond between ‘C’ and ‘Cl’ is a polar covalent bond whereas C-2 and C-1 bond (C-C bond) is expected to be nonpolar covalent bond. But, this bond acquires some polarity as chlorine is more electronegative than carbon. Chlorine pulls the bonding pair of electrons towards itself. Thus, the chlorine atom acquires a fractional negative charge, while the C-1 carbon atom acquires a fractional positive charge. As C-1 is further bonded to C-2, the positive polarity of C-1 pulls the shared pair of electrons of the C-2 – C-l bond more towards itself. As a result, a smaller positive charge is developed on C-2. Thus, the electron density gets displaced towards the chlorine atom not only along the [C-1 – Cl] bond, but also along the [C-2 – C-1] bond due to the inductive effect of Cl. This is represented as follows:
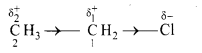
iii. The arrow head shown in the centre of the bond represents inductive effect. The direction of the arrow head indicates the direction of the permanent electron displacement along the sigma bond in the ground state.
iv. The inductive effect of an influencing group is transmitted along a chain of C-C bonds. However, this effect decreases rapidly with the increase in the number of intervening C-C single bonds and it becomes negligible beyond three C-C bonds.
![]()
v. The direction of the inductive effect of a bonded group depends upon whether electron density of the bond is withdrawn from the bonded carbon or donated by the bonded carbon. On the basis of this ability, the groups/substituents are classified as either electron withdrawing (accepting) or electron donating (releasing) groups with respect to hydrogen.
e.g. In chloroethane, Cl withdraws electron density from the carbon chain and is electron withdrawing. Therefore, chlorine is said to exert an electron withdrawing inductive effect or negative inductive effect (-I effect) on the carbon chain.
vi. a. Substituents or groups that shows -I effect: -Cl, -NO2, -CN, -COOH, -COOR, -OAr, etc.
b. Substituents or groups that shows +I effect: Alkyl groups such as -CH3, -CH2CH3, etc.
Question 70.
Consider the following molecules and answer the questions:
CH3 – CH2 – CH2 – Cl, CH3 – CH2 – CH2 – Br, CH3 – CH2 – CH2 – I.
i. What type of inductive effect is expected to operate in these molecules?
ii. Identify the molecules from these three, having the strongest and the weakest inductive effect.
Answer:
i. The groups responsible for inductive effect in these molecules are -Cl, -Br and -I, respectively. All these are halogen atoms which are more electronegative than carbon. Therefore, all of them exert -I effect, that is, electron withdrawing inductive effect.
ii. The -I effect of halogens is due to their electronegativity. A decreasing order of electronegativity in these halogens follows Cl > Br > I. Therefore, the strongest -I effect is expected in CH3 – CH2 – CH2 – Cl, while the weakest -I effect is expected for CH3 – CH2 – CH2 – I.
![]()
Question 71.
Which of the CH3 – CHCl2 and CH3CH2Cl is expected to have stronger -I effect?
Answer:
The group exerting -I effect is -Cl. In CH3CH2Cl, there is only one -Cl atom while in CH3 – CHCl2 there are two -Cl atoms. Therefore, CH3 – CHCl2 is expected to have strong -I effect.
Question 72.
Give an account of expected and observed values of carbon-carbon bond lengths in benzene.
Answer:
- In cyclic structure of benzene, three alternating C – C single bonds and C=C double bonds are present.
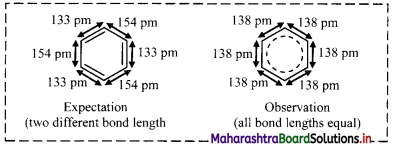
- Expected values of bond length of the C – C bond and C = C are 154 pm and 133 pm respectively.
- Experimental measurements show that benzene has a regular hexagonal shape and all the six carbon-carbon bonds have the same bond length of 138 pm, which is intermediate between C – C single bond and C=C double bond.
- This means that all the six carbon-carbon bonds in benzene are equivalent.
Note: Structure of benzene
Question 73.
What do you understand by the term conjugated system of π bonds?
Answer:
When Lewis structure of a compound has two or more multiple bonds alternating with single bonds, it is called a conjugated system of π bonds.
e.g. Benzene molecule
[Note: In such a system or in species having an atom carrying p orbital attached to a multiple bond, resonance theory is applicable.]
Question 74.
Identify the species that contains a conjugated system of π bonds. Explain your answer,
i. CH2 = CH – CH2 – CH = CH2
ii. CH2 = CH – CH = CH – CH3
Answer:
i. It does not contain conjugated system of π bonds, as the two C = C double bonds are separated by two C – C single bonds.
ii. It contains a conjugated system of π bonds, as the two C = C double bonds are separated by only one C – C single bond.
![]()
Question 75.
Explain in detail the important points of resonance theory.
Answer:
Resonance theory:
i. The π electrons in conjugated system of π bonds are not localized to a particular π bond.
ii. For a compound having a conjugated system of π bonds (or similar other systems), two or more Lewis structures are written by showing movement of π electrons (that is, delocalization of π electrons) using curved arrows.
The Lewis structures so generated are linked by double headed arrow and are called resonance structures or contributing structures or cononical structures of the species. Thus, two resonance structures can be drawn for benzene by delocalizing or shifting the π electrons :
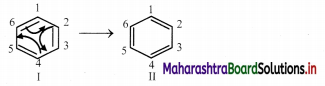
iii. The positions of the carbon atoms in the conjugated system of π bonds remain unchanged, but the positions of π electrons are different in different resonance structures.
e.g. In the resonance structure I of benzene there is a single bond between C1 and C2 while in the resonance structure II there is a double bond between C1 and C2.
iv. Any resonance structure is hypothetical and does not by itself represent any real molecule and can explain all the properties of the compound. The real molecule has, however, character of all the resonance structures those can be written. The real or actual molecule is said to be the resonance hybrid of all the resonance structures.
e.g. An actual benezene molecule is the resonance hybrid of structures I and II and exhibit character of both these structures. Its approximate representation can be shown as a dotted.circle inscribed in a regular hexagon. Thus, each carbon-carbon bond in benzene has single as well as double bond character and the ring has a regular hexagonal shape.

v. Hypothetical energy of an individual resonance structure can be calculated using bond energy values. The energy of actual molecule is, however, lower than that of any one of the resonance structures. In other words, resonance hybrid is more stable than any of the resonance structures. The difference in the actual energy and the lowest calculated energy of a resonance structure is called resonance stabilization energy or just resonance energy. Thus, resonance leads to stabilization of the actual molecule.
Question 76.
State the rules to be followed for writing resonating structures.
Answer:
Rules to be followed for writing resonating structures:
- Resonance structures can be written only when all the atoms involved in the n conjugated system lie in the same place.
- All the resonance structures must have the same number of unpaired electrons.
- Resonance structures contribute to the resonance hybrid in accordance to their energy or stability. More stable (having low energy) resonance structures contribute largely and thus are important.
Question 77.
What are the important points considered while selecting the most stable resonance structure if there are several contributing/resonance structures for a compound?
Answer:
When several resonance structures are compared, then the resonance structure is considered to be more stable if it has:
- more number of covalent bonds,
- more number of atoms with complete octet or duplet,
- less separation, if any, of opposite charges,
- negative charge, if any, on more electronegative atom and positive charge, if any, on more electropositive atom and
- more dispersal of charge.
[Note: When all the resonance structures of a species are equivalent to each other, the species is highly resonance stabilized. For example, R – COO-, \(\mathrm{CO}_{3}^{2-}\)]
Question 78.
Write resonance structures of H – COO– and comment on their relative stability.
Answer:
i. First the detailed bond structure of H – COO– showing all the valence electron is drawn and then other resonance structures are generated using curved arrow to show movement of π-electrons.
ii. Two resonance structures are written for H – COO–.
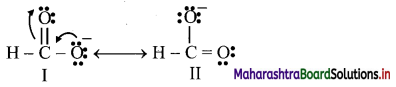
Both the resonance structures I and II are equivalent to each other, and therefore, are equally stable.
Question 79.
Identify the species which has resonance stabilization. Justify your answer.
![]()
Answer:
i. The bond structure shows that there is no π bond. Therefore, no resonance and no resonance stabilization.
ii.
N = O double b5itd is attached to ‘O’ which carries lone pair of electrons in a p orbital.
Therefore, resonance structures can be written as shown and species is resonance stabilized.
iii.
![]()
The Lewis structure shows two C = C double bonds alternating with a C – C single bond.
Therefore, resonance structures can be written as shown and the species is resonance stabilized.
![]()
Question 80.
Write three resonance structures for CH3 – CH = CH – CHO. Indicate their relative stabilities and explain.
Answer:
Three resonance structures are:
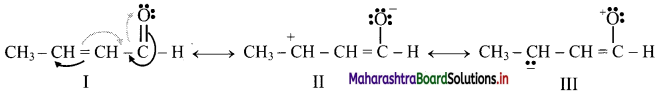
Stability order: I > II > III
I: Contains more number of covalent bonds, each carbon atom and oxygen atom has complete octet, and involves no separation of opposite charges. Therefore, the most stable resonance structure.
II: Contains one covalent bond less than in I, one carbon (C+) has only 6 valence electrons, involves separation of opposite charges; the resonance structure II has -ve charge on more electronegative ‘O’ and +ve charge on more electropositive ‘C’. It has intermediate stability.
III: Contains one covalent bond less than in I, oxygen has only 6 valence electrons, involves separation of opposite charge, has -ve charge on the more electropositive ‘C’ and +ve charge on more electronegative ‘O’. All these factors are unfavourable for stability. Therefore, it is the least stable.
Question 81.
Define: Resonance effect.
Answer:
The polarity produced in the molecule by the interaction between conjugated n bonds (or that between n bond and p orbital on attached atom) is called the resonance effect or mesomeric effect.
Question 82.
Explain in short:
i. Positive resonance (+R) effect
ii. Negative resonance (-R) effect
Answer:
i. Positive resonance (+R) effect or electron donating/releasing resonance effect:
a. If the substituent group has a lone pair of electrons to donate to the attached K bond or conjugated system of π bonds, the effect is called +R effect.
b. The +R effect increases electron density at certain positions in a molecule.
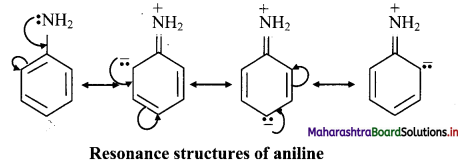
e.g. +R effect in aniline increases the electron density at ortho and para positions.
c. Halogen, -OH, -OR, -O–, -NH2, -NHR, -NR2, – NHCOR, -OCOR, etc. are the groups which show +R effect.
ii. Negative resonance (-R) effect:
a. If the substituent group has a tendency to withdraw electrons from the attached π bond or conjugated system of π bonds towards itself the effect is called -R effect.
b. The -R effect results in developing a positive polarity at certain positions in a molecule.
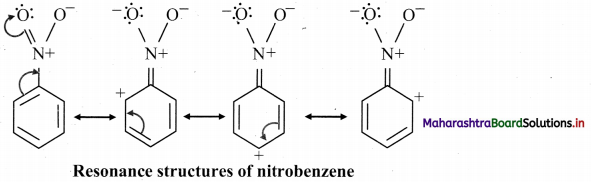
e.g. -R effect in nitrobenzene develops positive polarity at ortho and para positions.
c. -COOH, -CHO, – CO -, -CN, -NO2, -COOR, etc., are the groups which represent -R effect.
Question 83.
Draw resonance structures showing +R effect in aniline.
Answer:
The following resonance structures can be drawn for aniline:
Question 84.
Draw resonance structures showing -R effect in nitrobenzene.
Answer:
The following resonance structures can be drawn for nitrobenzene:
Question 85.
Write a note on electromeric effect.
Answer:
Electromeric effect:
i. This is a temporary electronic effect exhibited by multiple-bonded groups in the excited state in the presence of a reagent.
ii. When a reagent approaches a multiple bond, the electron pair gets completely shifted to one of the multiply, bonded atoms, giving a charge separated structure.
iii.

This effect is temporary and disappears when the reagent is removed from the reacting system.
![]()
Question 86.
Explain the term hyperconjugation in short.
Answer:
Hyperconjugation:
i. Hyperconjugation is a permanent electronic effect.
ii. It explains the stability of a carbocation, free radical or alkenes.
iii. It involves delocalization of sigma electrons of a C – H bond of an alkyl group directly attached to a carbon atom, which is part of an unsaturated system or has an empty p orbital or a p orbital with an unpaired electron.
iv. Following species are stabilized by resonance:
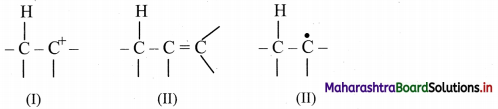
Question 87.
Explain hyperconjugation in ethyl carbocation.
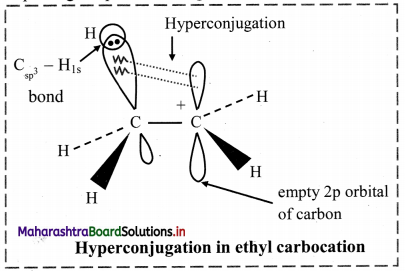
Answer:
i. In ethyl cation \(\mathrm{CH}_{3} \stackrel{+}{\mathrm{CH}}_{2}\), positively charged carbon atom is attached to a methyl group.
ii. The positively charged carbon atom has six electrons; it is sp2 hybridized and has an empty p orbital available for hyperconjugation.
iii. One of the C – H bonds of the methyl group can align in plane of the empty p orbital. The sigma electrons constituting the C – H bond can be delocalized into this empty p orbital.
iv. Therefore, hyperconjugation arises due to the partial overlap of a C-H bond with the empty p orbital of an adjacent positively charged carbon atom. Thus, hyperconjugation is a σ-π conjugation.
v. Hyperconjugation structures in ethyl carbocation can be represented as:
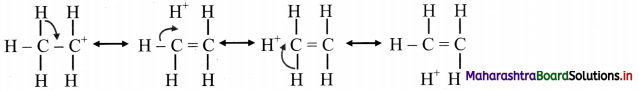
vi. In the contributing structures, there is no covalent bond shown between the carbon and one of the α-hydrogens. Hence, hyperconjugation is also called as ‘no bond resonance’.
vii. This type of overlap stabilizes the cation, because the electron density from the adjacent a bond helps in dispersing the positive charge.
Question 88.
Explain the stability of tert-butyl cation, isopropyl cation, ethyl cation and methyl cation on the basis of hyperconjugation.
Answer:
i. Greater the number of alkyl groups attached to a positively charged carbon atom, more is the number of α-hydrogens, more is the hyperconjugation structures and more is the stability of the cation.
ii. Thus, the relative stability of the cations decreases in the order:
3° carbocation > 2° carbocation > 1° carbocation > Methyl cation
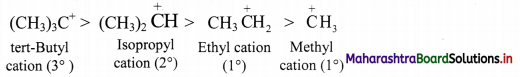
Question 89.
Explain hyperconjugation in propene.
Answer:
i. In propene, CH3 – CH = CH2, one of the sp2 hybridized carbon atom of the double bond is attached to sp3 hybridized carbon atom of methyl group.
ii. One of the C-H bonds of the methyl group can align in plane of the p orbital of sp2 hybridized C-atom and the electrons constituting the C-H bond in plane with this p orbital can then be delocalized into the p orbital.
iii. Therefore, hyperconjugation arises due to the partial overlap of a C-H bond with the p orbital of an adjacent sp2 hybridized carbon atom.
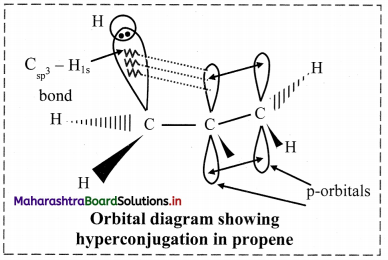
iv. Hyperconjugation (no bond resonance) structures for propene can be represented as:
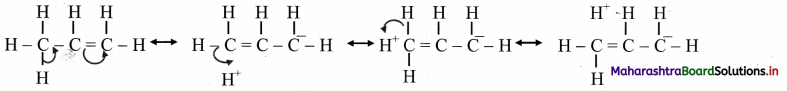
Question 90.
Write the Lewis dot structures of but-1-ene and but-2-ene? Also, write the bond line formula of both the compounds.
Answer:

Question 91.
Due to contamination by viruses, the hospital authorities had asked Ranjan, the ward boy, to keep cleaning the hospital lobby using some antiseptic. Ranjan would wipe the floor by adding Dettol to water and would always keep the premises clean. One of the active ingredients in Dettol is chloroxylenol (4-chloro-3,5-dimethylphenol). Ranjan was also actively associated with an NGO, which was involved in Swachh Bharat campaign. Based on this passage, answer the following questions.
i. Which functional groups are present in chloroxylenol?
ii. Write the bond line and molecular formula of chloroxylenol.
iii. Identify one group each in chloroxylenol which show +I and -I effect, respectively.
Answer:
i. chloroxylenol is
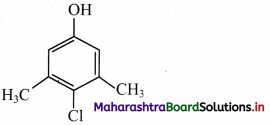
Functional groups present in chloroxylenol are chloro (-Cl) and phenolic -OH group.
ii. The bond line formula of chloroxylenol can be shown as,
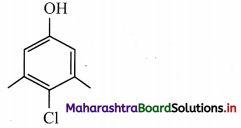
Its molecular formula is C8H9OCl or C8H8ClOH
iii. Group which shows +I effect = -CH3; group which shows -I effect = -Cl
![]()
Multiple Choice Questions
1. Which of the following method can be used to represent 3-D structure of organic molecules?
i. Wedge formula
ii. Fischer projection formula
iii. Newman projection formula
iv. Sawhorse formula
(A) Only ii and iii.
(B) Only i and iii.
(C) Only iii and iv.
(D) All of the above
Answer:
(D) All of the above
2. Which one is the INCORRECT statement?
(A) Open chain compounds are called aliphatic compounds.
(B) Unsaturated compounds contain multiple bonds in them.
(C) Saturated hydrocarbons are called alkenes.
(D) Aromatic compounds possess a characteristic aroma.
Answer:
(C) Saturated hydrocarbons are called alkenes.
3. Choose the INCORRECT statement from the following.
(A) Cyclohexane is an alicyclic compound.
(B) Pyridine is a heterocyclic compound.
(C) Piperidine is an aromatic compound.
(D) Tropone is a non-benzenoid compound.
Answer:
(C) Piperidine is an aromatic compound.
4. Which of the following is NOT a cyclic compound?
(A) Anthracene
(B) Pyrrole
(C) Phenol
(D) Neopentane
Answer:
(D) Neopentane
5. Which of the following is a cycloalkane?
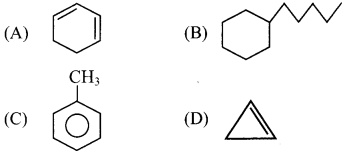
Answer:
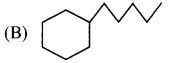
![]()
6. Which one of the following could be a cyclic alkane?
(A) C5H5
(B) C3H6
(C) C4H6
(D) C2H6
Answer:
(B) C3H6
7. Which of the following is a heterocyclic compound?
(A) Naphthalene
(B) Thiophene
(C) Phenol
(D) Aniline
Answer:
(B) Thiophene
8. Which of the following is NOT aromatic?
(A) Benzene
(B) Toluene
(C) Cyclopentane
(D) Phenol
Answer:
(C) Cyclopentane
9. Cyclohexene is …………….
(A) aromatic
(B) alicyclic
(C) benzenoid
(D) aliphatic
Answer:
(B) alicyclic
10. An organic compound ‘X’ (molecular formula C6H7O2N) has six carbons in a ring system, two double bonds and also a nitro group as a substituent, ‘X’ is …………..
(A) homocyclic and aromatic
(B) homocyclic but not aromatic
(C) heterocyclic
(D) aromatic but not homocyclic
Answer:
(B) homocyclic but not aromatic
![]()
11. Which of the following structure represents an aldehyde?
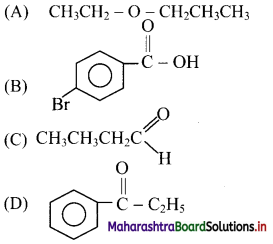
Answer:
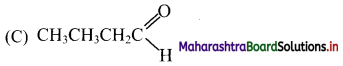
12. A member of a homologous series differs from immediate above or below member by …………… group.
(A) – CH3
(B) – CH2 –
(C) – CH2CH3
(D) – C6H5 –
Answer:
(B) – CH2 –
13. Which of the following is NOT a branched chain alkyl group?
(A) Isobutyl group
(B) n-Butyl group
(C) sec-Butyl group
(D) tert-Butyl group
Answer:
(B) n-Butyl group
14. In IUPAC nomenclature, the number which indicates the position of the substituent is called ………….
(A) locant
(B) delocant
(C) prefix
(D) suffix
Answer:
(A) locant
15. The IUPAC name of the following compound is …………..
(A) 1,1 -dimethyl-2-ethylcyclohexane
(B) 2-ethyl-1,1 -dimethylcyclohexane
(C) 1 -ethyl-2,2-dimethylcyclohexane
(D) 2,2-dimethyl-1-ethylcyclohexane
Answer:
(B) 2-ethyl-1,1 -dimethylcyclohexane
![]()
16. Which is the CORRECT name of ………….
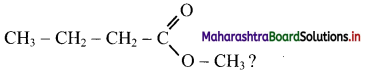
(A) Propyl ethanoate
(B) Ethyl propanoate
(C) Methyl butanoate
(D) Butyl methanoate
Answer:
(C) Methyl butanoate
17. Homolytic fission is NOT favourable in presence of …………..
(A) UV light
(B) catalyst like peroxide
(C) polar solvent
(D) high temperature
Answer:
(C) polar solvent
18. The total number of electrons in the carbon atom of methyl free radical is ………….
(A) six
(B) seven
(C) eight
(D) nine
Answer:
(B) seven
19. The most unstable carbocation amongst the following is ……………
(A) (CH3)3C+
(B) (CH3)2CH+
(C) CH3 – CH2+
(D) CH3+
Answer:
(D) CH3+
20. Which of the following represents a pair of electrophiles?
(A) BF3, H2O
(B) AlCl3, NH3
(C) CN–, ROH
(D) BF3, AlCl3
Answer:
(D) BF3, AlCl3
![]()
21. This group shows +I effect.
(A) -Br
(B) -CN
(C) -COOH
(D) -CH2CH3
Answer:
(D) -CH2CH3
22. Which of the following group shows negative resonance effect?
(A) -O-
(B) -COOH
(C) -NHCOR
(D) -NH2
Answer:
(B) -COOH
23. Resonance is NOT exhibited by ………….
(A) phenol
(B) aniline
(C) nitrobenzene
(D) cyclohexane
Answer:
(D) cyclohexane
24. All bonds in benzene are equal due to ………….
(A) tautomerism
(B) metamerism
(C) resonance
(D) isomerism
Answer:
(C) resonance