Balbharti Maharashtra State Board 11th Chemistry Textbook Solutions Chapter 7 Modern Periodic Table Textbook Exercise Questions and Answers.
Maharashtra State Board 11th Chemistry Solutions Chapter 7 Modern Periodic Table
1. Explain the following
Question A.
The elements Li, B, Be and N have the electronegativities 1.0, 2.0, 1.5, and 3.0, respectively on the Pauling scale.
Answer:
- Li, B, Be and N belong to the same period.
- As we move across a period from left to right in the periodic table, the effective nuclear charge increases steadily and therefore, electronegativity increases.
Hence, the elements Li, B, Be and N have the electronegativities 1.0, 2.0, 1.5, and 3.0, respectively on the Pauling scale.
Question B.
The atomic radii of Cl, I and Br are 99, 133 and 114 pm, respectively.
Answer:
- Cl, I and Br belong to group 17 (halogen group) in the periodic table.
- As we move down the group from top to bottom in the periodic table, a new shell gets added in the atom of the elements.
- As a result, the effective nuclear charge decreases due to increase in the atomic size as well as increased shielding effect.
- Therefore, the valence electrons experience less attractive force from the nucleus and are held less tightly resulting in the increased atomic radius.
- Thus, their atomic radii increases in the following order down the group.
Cl (99 pm) < Br (114 pm) < I (133 pm)
Hence, the atomic radii of Cl, I and Br are 99, 133 and 114 pm, respectively.
Question C.
The ionic radii of F– and Na+ are 133 and 98 pm, respectively.
Answer:
- F– and Na+ are isoelectronic ions as they both have 10 electrons.
- However, the nuclear charge on F– is +9 while that of Na+ is +11.
- In isoelectronic species, larger nuclear charge exerts greater attraction on the electrons and thus, the radius of that isoelectronic species becomes smaller.
Thus, F– has larger ionic radii (133 pm) than Na+ (98 pm).
![]()
Question D.
13Al is a metal, 14Si is a metalloid and 15P is a nonmetal.
Answer:
- Electronic configuration of Al is [Ne] 3s2 3p1, 14Si is [Ne] 3s2 3p2 and that of 15P is [Ne] 3s2 3p3.
- Metals are characterized by the ability to form compounds by loss of valence electrons.
- ‘Al’ has 3 valence electrons, thus shows tendency to lose 3 valence electrons to complete its octet. Hence, Al is a metal.
- Nonmetals are characterized by the ability to form compounds by gain of valence electrons in valence shell.
- ‘P’ has 5 valence electrons thus, shows tendency to gain 3 electrons to complete its octet. Hence, ‘P’ is a nonmetal.
- Si has four valence electrons, thus it can either lose/gain electrons to complete its octet. Hence, behaves as a metalloid.
Question E.
Cu forms coloured salts while Zn forms colourless salts.
Answer:
- Electronic configuration of 29CU is [Ar] 3d104s1 while that of Zn is [Ar] 3d104s2.
- Electronic configuration of Cu in its +1 oxidation state is [Ar] 3d10 while that in +2 oxidation state is [Ar] 3d9.
- Therefore, Cu contains partially filled d orbitals in +2 oxidation state and thus, Cu2+ salts are coloured.
- However, Zn has completely filled d orbital which is highly stable and hence, it does not form coloured ions.
Hence, Cu forms coloured salts while Zn forms colourless salts.
2. Write the outer electronic configuration of the following using orbital notation method. Justify.
A. Ge (belongs to period 4 and group 14)
B. Po (belongs to period 6 and group 16)
C. Cu (belongs to period 4 and group 11)
Answer:
A. a. Ge belongs to period 4. Therefore, n = 4.
b. Group 14 indicates that the element belongs to the p-block of the modem periodic table.
c. The general outer electronic configuration of group 14 elements is ns2 np2.
d. Thus, the outer electronic configuration of Ge is 4s2 4p2.
B. a. Po belongs to period 6. Therefore, n = 6.
b. Group 16 indicates that the element belongs to the p-block of the modem periodic table.
c. The general outer electronic configuration of group 16 elements is ns2 np4.
d. Thus, the outer electronic configuration of Po is 6s2 6p4.
C. a. Cu belongs to period 4. Therefore, n = 4.
b. Group 11 indicates that the element belongs to the d-block of the modem periodic table.
c. The general outer electronic configuration of the d-block elements is ns0-2(n-1)d1-10.
d. The expected configuration of Cu is 4s23d9. However, the observed configuration of Cu is 4s13d10. This is due to the extra stability associated with completely filled d-subshell. Thus, the outer electronic configuration of Cu is 4s13d10.
![]()
3. Answer the following
Question A.
La belongs to group 3 while Hg belongs to group 12 and both belong to period 6 of the periodic table. Write down the general outer electronic configuration of the ten elements from La to Hg together using orbital notation method.
Answer:
i. La and Hg both belongs to period 6. Therefore, n = 6.
ii. Elements of group 3 to group 12 belong to the d-block of the modem periodic table.
iii. The general outer electronic configuration of the d-block elements is ns0-2 (n -1 )1-10.
iv. Therefore, the outer electronic configuration of all ten elements from La to Hg is as given in the table below.

[Note: There are 14 elements between La and Hf which are called lanthanides. Therefore, after La, electrons are filled in 4f subshell of lanthanide elements. Once all the 14 elements of lanthanide series are filled, next electron enters 5d subshell of Hf. Hence, the outer electronic configurations of Hf to Hg often include completely filled 4f subshell. For example, the electronic configuration of Hf ‘5d26s2’ can also be written as ‘4f145d26s2’.]
Question B.
Ionization enthalpy of Li is 520 kJ mol-1 while that of F is 1681 kJ mol-1. Explain.
Answer:
- Both Li and F belong to period 2.
- Across a period, the screening effect is the same while the effective nuclear charge increases.
- As a result, the outer electron is held more tightly and therefore, the ionization enthalpy increases across a period.
- Hence, F will have higher ionization enthalpy than Li.
Thus, ionization enthalpy of Li is 520 kJ mol-1 while that of F is 1681 kJ mol-1.
Question C.
Explain the screening effect with a suitable example.
Answer:
i. In a multi-electron atom, the electrons in the inner shells tend to prevent the attractive influence of the nucleus from reaching the outermost electron.
ii. Thus, they act as a screen or shield between the nuclear attraction and outermost or valence electrons. This effect of the inner electrons on the outer electrons is known as screening effect or shielding effect.
iii. Across a period, screening effect due to inner electrons remains the same as electrons are added to the same shell.
iv. Down the group, screening effect due to inner electrons increases as a new valence shell is added.
e.g. Potassium (19K) has electronic configuration 1s22s22p63s23p64s1.
K has 4 shells and thus, the valence shell electrons are effectively shielded by the electrons present in the inner three shells. As a result of this, valence shell electron (4s1) in K experiences much less effective nuclear charge and can be easily removed.
![]()
Question D.
Why the second ionization enthalpy is greater than the first ionization enthalpy ?
Answer:
The second ionization enthalpy (ΔiH2) is greater than the first ionization enthalpy (ΔiH1) as it involves removal of electron from the positively charged species.
Question E.
Why the elements belonging to the same group do have similar chemical properties ?
Answer:
- Chemical properties of elements depend upon their valency.
- Elements belonging to the same group have the same valency.
Hence, the elements belonging to the same group show similar chemical properties.
Question F.
Explain : electronegativity and electron gain enthalpy. Which of the two can be measured experimentally?
Answer:
i. The ability of a covalently bonded atom to attract the shared electrons toward itself is called electronegativity (EN). Electronegativity cannot be measured experimentally. However, various numerical scales to express electronegativity were developed by many scientists. Pauling scale of electronegativity is the one used most widely.
ii. Electron gain enthalpy is a quantitative measure of the ease with which an atom adds an electron forming the anion and is expressed in kJ mol-1. Thus, it is an experimentally measurable quantity.
4. Choose the correct option
Question A.
Consider the elements B, Al, Mg and K predict the correct order of metallic character :
a. B > Al > Mg > K
b. Al > Mg > B > K
c. Mg > Al > K > B
d. K > Mg > Al > B
Answer:
d. K > Mg > Al > B
Question B.
In modern periodic table, the period number indicates the :
a. atomic number
b. atomic mass
c. principal quantum number
d. azimuthal quantum number
Answer:
c. principal quantum number
Question C.
The lanthanides are placed in the periodic table at
a. left hand side
b. right hand side
c. middle
d. bottom
Answer:
d. bottom
![]()
Question D.
If the valence shell electronic configuration is ns2np5, the element will belong to
a. alkali metals
b. halogens
c. alkaline earth metals
d. actinides
Answer:
b. halogens
Question E.
In which group of elements of the modern periodic table are halogen placed ?
a. 17
b. 6
c. 4
d. 2
Answer:
a. 17
Question F.
Which of the atomic number represent the s-block elements ?
a. 7, 15
b. 3, 12
c. 6, 14
d. 9, 17
Answer:
b. 3, 12
Question G.
Which of the following pairs is NOT isoelectronic ?
a. Na+ and Na
b. Mg2+ and Ne
c. Al3+ and B3+
d. P3– and N3-
Answer:
b. Mg2+ and Ne
Question H.
Which of the following pair of elements has similar properties ?
a. 13, 31
b. 11, 20
c. 12, 10
d. 21, 33
Answer:
a. 13, 31
![]()
5. Answer the following questions
Question A.
The electronic configuration of some elements are given below:
a. 1s2
b. 1s22s22p6
In which group and period of the periodic table they are placed ?
Answer:
a. 1s2
Here n = 1. Therefore, the element belongs to the 1st period.
The outer electronic configuration 1s2 corresponds to the maximum capacity of 1s, the complete duplet. Therefore, the element is placed at the end of the 1st period in the group 18 of inert gases in the modem periodic table,
b. 1s22s22p6
Here n = 2. Therefore, the element belongs to the 2nd period.
The outer electronic configuration 2s22p6 corresponds to complete octet. Therefore, the element is placed in the 2nd period of group 18 in the modem periodic table.
Question B.
For each of the following pairs, indicate which of the two species is of large size :
a. Fe2+ or Fe3+
b. Mg2+ or Ca2+
Answer:
a. Fe2+ has a larger size than Fe3+.
b. Ca2+ has a larger size than Mg2+.
Question C.
Select the smaller ion form each of the following pairs:
a. K+, Li+
b. N3-, F–
Answer:
i. Li+ has smaller ionic radius than K+
ii. F– has smaller ionic radius than N3-.
Question D.
With the help of diagram answer the questions given below:
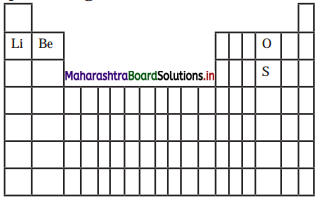
a. Which atom should have smaller ionization enthalpy, oxygen or sulfur?
b. The lithium forms +1 ions while berylium forms +2 ions ?
Answer:
Sulfur should have smaller ionization energy than oxygen.
a. Lithium has electronic configuration 1s22s1 while that of beryllium is 1s22s2.
b. Li can achieve a noble gas configuration by losing one electron while Be can do so by losing two electrons. Hence, lithium forms +1 ions while beryllium forms +2 ions.
![]()
Question E.
Define : a. Ionic radius
b. Electronegativity
Answer:
a. Ionic radius: Ionic radius is defined as the distance of valence shell of electrons from the centre of the nucleus in an ion.
b. Electronegativity: The ability of a covalently bonded atom to attract the shared electrons toward itself is called electronegativity (EN).
Question F.
Compare chemical properties of metals and non-metals.
Answer:
i. Metals (like alkali metals) react vigorously with oxygen to form oxides which reacts with water to form strong bases.
e. g. Sodium (Na) reacts with oxygen to form Na2O which produces NaOH on reaction with water.
ii. Nonmetals (like halogens) react with oxygen to form oxides which on reaction with water form strong acids.
e.g. Chlorine reacts with oxygen to form Cl2O7 which produces HClO4 on reaction with water.
Question G.
What are the valence electrons ? For s-block and p-block elements show that number of valence electrons is equal to its group number.
Answer:
- Electrons present in the outermost shell of the atom of an element are called valence electrons.
- 3Li is an s-block element and its electronic configuration is 1s22s1. Since it has one valence electron, it is placed in group 1.
- Therefore, for s-block elements, group number = number of valence electrons.
- However, for p-block elements, group number = 18 – number of electrons required to attain complete octet.
- 7N is a p-block element and its electronic configuration is 1s22s22p3. Since it has five electrons in its valence shell, it is short of three electrons to complete its octet.
- Therefore, its group number = 18 – 3 = 15.
Question H.
Define ionization enthalpy. Name the factors on which ionisation enthalpy depends? How does it vary down the group and across a period?
Answer:
i. The energy required to remove an electron from the isolated gaseous atom in its ground state is called ionization enthalpy (ΔiH).
Ionization enthalpy is the quantitative measure of tendency of an element to lose electron and expressed in kJ mol-1.
ii. Ionization energy depends on the following factors
- Size (radius) of an atom
- Nuclear charge
- The shielding or screening effect of inner electrons
- Nature of electronic configuration
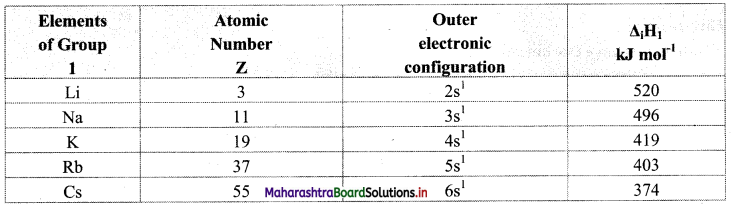
iii. Variation of ionization energy down the group: On moving down the group, the ionization enthalpy decreases. This is because electron is to be removed from the larger valence shell. Screening due to core electrons goes on increasing and the effective nuclear charge decreases down the group. As a result, the removal of the outer electron becomes easier down the group.
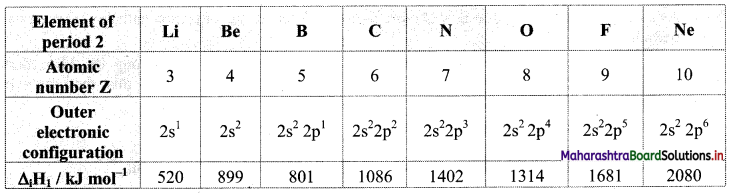
iv. Variation of ionization energy across a period: The screening effect is the same while the effective nuclear charge increases across a period. As a result, the outer electron is held more tightly and hence, the ionization enthalpy increases across a period. Therefore, the alkali metal shows the lowest first ionization enthalpy while the inert gas shows the highest first ionization enthalpy across a period.
Note: First ionization enthalpy values of elements of group 1.
Note: First ionization enthalpy values of elements of period 2.
![]()
Question I.
How the atomic size vary in a group and across a period? Explain with suitable example.
Answer:
i. Variation in atomic size down the group:
a. As we move down the group from top to bottom in the periodic table, the atomic size increases with the increase in atomic number.
b. This is because, as the atomic number increases, nuclear charge increases but simultaneously the number of shells in the atoms also increases.
c. Asa result, the effective nuclear charge decreases due to increase in the size of the atom and shielding effect increases down the group. Thus, the valence electrons experience less attractive force from nucleus and are held less tightly.
d. Hence, the atomic size increases in a group from top to bottom.
e. g.
- In group 1, as we move from top to bottom i.e., from Li to Cs, a new shell gets added in the atom of the elements and the electrons are added in this new shell.
- As a result of this, the effective nuclear charge goes on decreasing and screening effect goes on increasing down a group.
- Therefore, the atomic size is the largest for Cs and is the smallest for Li in group 1.
[Note: Atomic radii of Li and Cs are 152 pm and 262 pm respectively.]
ii. Variation in atomic size across a period:
a. As we move across a period from left to right in the periodic table, the atomic size of an element decreases with the increase in atomic number.
b. This is because, as the atomic number increases, nuclear charge increases gradually but addition of electrons takes place in the same shell.
c. Therefore, as we move across a period, the effective nuclear charge increases but screening effect caused by the core electrons remains the same.
d. As a result of this, attraction between the nucleus and the valence electrons increases. Therefore, valence electrons are more tightly bound and hence, the atomic radius goes on decreasing along a period resulting in decrease in atomic size.
e. g.
- In the second period, as we move from left towards right i.e., from Li to F, the electrons are added in the second shell of all the elements in second period (except noble gas Ne).
- As a result of this, the effective nuclear charge goes on increasing from Li to F, however, screening effect remains the same.
- Therefore, the atomic size is the largest for Li (alkali metal) and is the smallest for F (halogen).
[Note: Atomic radii of Li and F are 152 pm and 64 pm respectively.]
Question J.
Give reasons.
a. Alkali metals have low ionization energies.
b. Inert gases have exceptionally high ionization energies.
c. Fluorine has less electron affinity than chlorine.
d. Noble gases possess relatively large atomic size.
Answer:
a. i. Across a period, the screening effect is the same while the effective nuclear charge increases.
ii. As a result, the outer electron is held more tightly and hence, the ionization enthalpy increases across a period.
iii. Since the alkali metals are present in the group 1 of the modem periodic table, they have low ionization energies.
b. i. Across a period, the screening effect is the same and the effective nuclear charge increases.
ii. As a result, the outer electron is held more tightly and hence, the ionization enthalpy increases across a period.
iii. Inert gases are present on the extreme right of the periodic table i.e., in group 18. Also, inert gases have stable electronic configurations i.e., complete octet or duplet. Due to this, they are extremely stable and it is very difficult to remove electrons from their valence shell.
Hence, inert gases have exceptionally high ionization potential.
c. The less electron affinity of fluorine is due to its smaller size. Adding an electron to the 2p orbital in fluorine leads to a greater repulsion than adding an electron to the larger 3p orbital of chlorine.
Hence, fluorine has less electron affinity than chlorine.
d. i. Noble gases have completely filled valence shell i.e., complete octet (except He with complete duplet).
ii. Since their valence shell contains eight electrons, they experience greater electronic repulsion and this results in increased atomic size (atomic radii) of the noble gas elements.
Hence, noble gases possess
Question K.
Consider the oxides Li2O, CO2, B2O3.
a. Which oxide would you expect to be the most basic?
b. Which oxide would be the most acidic?
c. Give the formula of an amphoteric oxide.
Answer:
a. Li2O is the most basic oxide.
b. CO2 is the most acidic oxide.
c. Formula of an amphoteric oxide: Al2O3.
[Note: Both B2O3 and CO2 are acidic oxides. But CO2 is more acidic oxide as compared to B2O3. Hence, CO2 is most acidic oxide amongst the given.]
Activity :
Question 1.
Prepare a wall mounting chart of the modern periodic table.
Answer:
Students can scan the adjacent Q.R. Code to visualise the modern periodic table and are expected to prepare the chart on their own.

![]()
11th Chemistry Digest Chapter 7 Modern Periodic Table Intext Questions and Answers
Can you recall? (Textbook Page No. 93)
Question 1.
What was the basis of classification of elements before the knowledge of electronic structure of atom?
Answer:
Elements were classified on the basis of their physical properties before the knowledge of electronic structure of atom.
Question 2.
Name the scientists who made the classification of elements in the nineteenth century.
Answer:
Dmitri Mendeleev, John Newlands and Johann Doberiener were the scientists who made the classification of elements based on their atomic mass in the nineteenth century.
Question 3.
What is Mendeleev’s periodic law?
Answer:
Mendeleev’s periodic law: “The physical and chemical properties of elements are the periodic function of their atomic masses
Question 4.
How many elements are discovered until now?
Answer:
Including manmade elements, total 118 elements are discovered until now.
Question 5.
How many horizontal rows and vertical columns are present in the modern periodic table?
Answer:
The modem periodic table consists of seven horizontal rows called periods numbered from 1 to 7 and eighteen vertical columns called groups numbered from 1 to 18.
Just think. (Textbook Page No. 93)
Question 1.
How many days pass between two successive full moon nights?
Answer:
29.5 days i.e., approximately 30 days pass between two successive full moon nights.
Question 2.
What type of motion does a pendulum exhibit?
Answer:
A pendulum exhibits periodic motion since it traces the same path after regular interval of time.
Question 3.
Give some other examples of periodic events.
Answer:
Following are some other examples of periodic events:
- Motion of earth around the sun.
- Rotation of earth around its own axis.
- Day and night.
![]()
Can you recall? (Textbook Page No. 95)
Question i.
What does the principal quantum number ‘n’ and azimuthal quantum number ‘l’ of an electron belonging to an atom represent?
Answer:
The principal quantum number ‘n’ represents the outermost or valence shell of an element (which corresponds to period number) while azimuthal quantum number ‘l’ constitutes a subshell belonging to the shell for the given ‘n’.
Question ii.
Which principle is followed in the distribution of electrons in an atom?
Answer:
The distribution of electrons in an atom is according to the following three principles:
- Aufbau principle
- Pauli’s exclusion principle
- Hund’s rule of maximum multiplicity
[Note: According to aufbau principle, electrons are filled in the subshells in the increasing order of their energies which follows the following order: s < p < d < f.]