Balbharti Maharashtra State Board 12th Chemistry Important Questions Chapter 7 Elements of Groups 16, 17 and 18 Important Questions and Answers.
Maharashtra State Board 12th Chemistry Important Questions Chapter 7 Elements of Groups 16, 17 and 18
Question 1.
What are p-block elements?
Answer:
The elements in which the differentiating electron (last filling electron) enters the p-orbital of the outermost shell of the atoms are called p-block elements. The elements of groups 16, 17 and 18 are p-block elements.
![]()
Question 2.
To which groups in the periodic table do p-block elements belong?
Answer:
A maximum of 6 electrons can be accommodated in the p-subshell, giving rise to six groups. In the periodic table, groups 13 to 18 include p-block elements.
Question 3.
What is the general electronic configuration of p-block elements?
Answer:
The general electronic configuration of valence shell of p-block elements is ns2 np1 – 6 (except He which has electronic configuration Is2).
Question 4.
What factors influence the properties of p-block elements?
Answer:
The properties of p-block elements are influenced by
- atomic and ionic radii
- ionisation enthalpy
- electron gain enthalpy (or electron affinity)
- electronegativity
- the presence or absence of electrons in d or d and orbitals in the p-block elements. m
Question 5.
Mention the symbols and atomic numbers of p-block elements. Also show the positions in the periods and groups.
Answer:
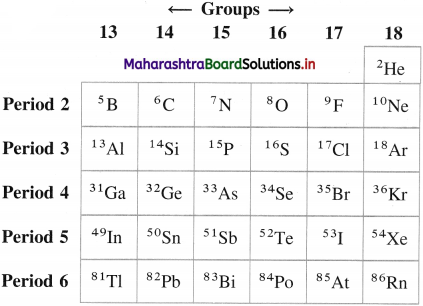
Question 6.
Name the elements in groups 16, 17 and 18. What is the oxygen family?
Answer:
Group 16 elements :
Oxygen (8O); Sulphur (16S); Selenium (34Se); Tellurium(52Te); Polonium(84Po)
Group 17 elements :
Fluorine (9F); Chlorine (17Cl); Bromine (35Br); Iodine (53I); Astatine (85At).
Group 18 elements :
Helium : (2He) Neon (10Ne), Argon (18Ar), Krypton (36Kr), Xenon(54Xe), Radon (86Rn)
The group 16 is called the oxygen family.
Question 7.
What are chalcogens?
Answer:
Group 16 elements are called chalcogens or ore-forming elements, as a large number of metal ores are oxides or sulphides.
Question 8.
Which is the most abundant element on the earth?
Answer:
(i) Oxygen is the most abundant of all the elements on the earth.
(ii) 46.6% by mass of earth’s crust contains oxygen and oxygen forms 20.95 % by volume of air.
Question 9.
How does sulphur occur?
Answer:
Sulphur forms 0.034 % by mass of the earth’s crust. In the combined form it occurs as follows :
- Sulphides : Galena (PbS), Zinc blende (ZnS), Copper pyrites (CuFeS2)
- Sulphates : Gypsum (CaSO4.2H2O), Epsom salt (MgSO4.7H2O), Baryte (BaSO4)
![]()
Question 10.
How do selenium, tellurium and poloniumoccur?
Answer:
Selenium and tellurium are found as metal selenides and tellurides in sulphide ores. Polonium occurs in nature as a decay product of thorium and uranium metals.
Question 11.
What are group 17 elements called?
Answer:
Group 17 elements are collectively called halogens. In Greek, halo means salt and gene means born, so halogens are salt produClng elements.
Question 12.
Why are halogens not found in the free state?
Answer:
Halogens are highly reactive due to high elec-tronegativities, hence do not occur in the free state. They mostly occur in the form of compounds.
Question 13.
How do group 17 elements occur 1
Answer:
Group 17 elements occur mostly in the form of compounds.
- Fluorine is available as insoluble fluorides in the earth’s crust.
- The important minerals are fluorspar, CaF2, cryolite, Na3AlF6, fluorapatite 3Ca3(PO4)2.CaF2.
- Halides (chloride, bromides, iodides) of Na, K, Mg and Ca are present in sea water.
- The dried up sea beds contain sodium chloride and the mineral carnallite (KCl.MgCl2.6H20).
- Seaweeds contain up to 0.5 % of iodine. The compound chile saltpetre contains 0.2% of sodium iodate.
- Astatine is the last member of the halogen family. It is radioactive and has a half life of 8.1 hours.
Question 14.
How do noble gases occur?
Answer:
- The most important source of noble gases is the atmosphere where they make up about 1% by volume of air. Argon is the major constituent.
- All noble gases occur in nature except Radon. Radon is a decay product of radioactive 226Ra.
- Helium on a large scale is obtained from natural gas. Helium and neon are found in the minerals pitchblende, monazite and cleveite.
- Xenon and Radon are the rarest noble gases.
Question 15.
How does the general electronic configuration of groups 16, 17 and 18 vary?
Answer:
The general electronic configuration of group 16 elements is ns2np4, group 17 is ns2np5 and group 18 is ns2np6. Group 16 has two electrons less than the stable electronic configuration of the nearest noble gas. while group 17 has one electron less than the stable electronic configuration of the nearest noble gas.
Question 16.
Give the names, atomic numbers and electronic configuration of the group 16 elements.
Answer:
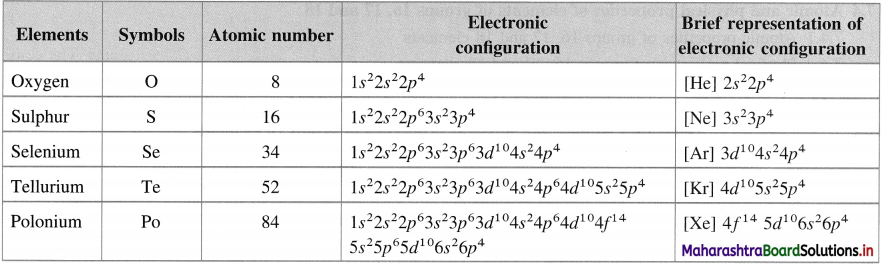
Question 17.
Write the names and electronic configuration of group 17 elements.
Answer:
Electronic configuration of group 17 elements

![]()
Question 18.
Write the names of group 18 elements and their electronic configuration.
Answer:
Electronic configuration of group 18 elements
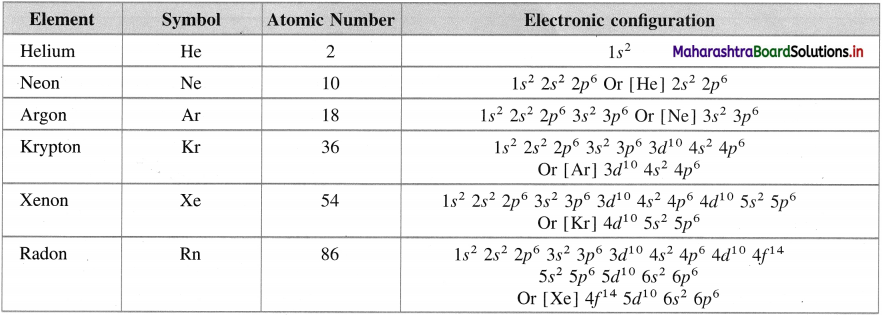
Question 19.
Mention the different atomic and physical properties of group 16 elements.
Answer:
Atomic and physical properties of group 16 elements

Question 20.
Mention the different atomic and physical properties of group 17 elements.
Answer:
Atomic and physical properties of group 17 elements

![]()
Question 21.
Mention the different atomic and physical properties of group 18 elements.
Answer:
Atomic and physical properties of group 18 elements

Question 22.
Discuss the trends in the following in case of groups 16, 17 and 18 elements.
(1) Atomic and ionic radii
(2) Ionisation enthalpy
(3) Electronegativity
(4) Electron gain enthalpy
Answer:
- Atomic and ionic radii :In groups 16, 17, 18 the atomic and ionic radii increase down the group, due to increase in the number of quantum shells. Across a period atomic or ionic radii decrease due to increase in effective nuclear charge.
- Ionisation enthalpy :
- The elements of groups 16, 17 and 18 have a high ionisation enthalpy
- In groups 16, 17, 18 the ionisation enthalpy decreases down the group, due to increase in atomic size.
- Electronegativity : In groups 16, 17, 18 the elec-tronegativity decreases down the group.
- Electron gain enthalpy :
- In groups 16 and 17 the electron gain enthalpy becomes less negative down the group.
- Group 18 elements have large positive electron gain enthalpy.
Question 23.
Explain the electron gain enthalpy of group 16 elements.
Answer:
- Electron gain enthalpy (or electron affinity) is the energy released when an electron is added to the valence shell of a gaseous atom forming gaseous ion.
\(\mathrm{M}_{(\mathrm{g})}+\mathrm{e}^{-} \longrightarrow \mathrm{M}^{+}{ }_{(\mathrm{g})}+\text { energy }\)
More the energy released more is electron gain enthalpy or electron affinity. - Group 16 elements have high values for electron gain enthalpy. On moving down the group, electron gain enthalpy decreases from S to Po.
- Oxygen has less negative electron gain enthalpy, due to high electronegativity, low atomic size and high electron density so that the incoming electron is repelled.
- Electron gain enthalpy of the elements decreases down the group due to successive decrease in electronegativity and nuclear attraction and increase in atomic size.
| Element | S | Se | Te | Po | O |
| Electron gain enthalpy kJ/mol | -200 | -195 | – 190 | – 174 | – 141 |
![]()
Question 24.
Oxygen has low electron gain enthalpy in group 16 elements. Explain.
Answer:
- Oxygen has low atomic size.
- It has high electronegativity (3.5).
- It has high electron density.
- The incoming electron is repelled due to high electron density hence oxygen has less negative value of electron gain enthalpy as compared to other group 16 elements. O(g) + e– → O–(g) ΔegH = – 141 kJ mol-1
Question 25.
Bromine has the highest negative electron gain enthalpy as compared to other elements of 4th period. Explain.
Answer:
- As compared to other elements (K to Kr) of 4th period, bromine (Br) has the lowest atomic size.
- It has electronic configuration, 35Br [Ar]18 3d10 4s2 4p5.
- It has seven valence electrons and it needs one electron to complete octet.
- Hence Br has the strongest tendency to gain an electron and energy released is maximum in the period. Br(g) + e– → Br–(g) ΔegH = -325 kJ mol-1
Therefore Br has the highest electron gain enthalpy as compared to other elements of 4th period.
Question 26.
Fluorine has higher electronegativity but less electron gain enthalpy. Explain.
Answer:
- Halogens have the highest values for electronegativity due to their small atomic radii and high nuclear charge.
- Fluorine has the highest electronegativity due to its small size.
- In fluorine due to strong inter electronic repulsions in the relatively small 2p orbitals and higher electron density, there is not much attraction of the nucleus for the incoming electron.
- Thus the electron gain enthalpy of fluorine is less in spite of higher electronegativity.
Question 27.
Explain the trend in atomic radius of group 18 elements.
Answer:
- The atomic radii of group 18 elements is larger than the atomic radii of group 17 elements.
- Down the group from He to Xe, atomic radii increases due to increase in the number of quantum shells.
- The atomic radii increase in the order He < Ne < Ar < Kr < Xe
Question 28.
Explain the variation in (1) ionisation enthalpy and (2) electron gain enthalpy in group 18 elements.
Answer:
(1) Ionisation enthalpy :
- In general, group 18 elements have high values of ionisation enthalpy.
- In a period, each noble gas has the highest ionisation enthalpy.
- The noble gases have electronic configuration, ns2 np6, they have complete octet with paired electrons and very stable closed-shell electronic configuration. Therefore high energy is required to remove the electron from valence shell.
- Down the group ionisation enthalpy decreases.
(2) Electron gain enthalpy :
- Group 18 elements have electronic configuration ns2 np6 and complete octet of electrons, hence they have no tendency to accept electrons.
- Therefore, they have a large positive electron gain enthalpy.
![]()
Question 29.
How does the atomic radii and ionisation enthalpy vary across a period?
Answer:
- The atomic or ionic radii decreases across a period with increase in atomic number and increase in the effective nuclear charge (Zeff).
- The ionisation enthalpy also increases across a period with increase in atomic number. This is due to addition of electrons to the same shell, while moving across a period.
- Inert element at the end of period has the highest value of ionisation enthalpy.
Question 30.
Why are atomic radii of noble gases larger than those of halogens?
Answer:
- Halogens (F to At) are in group 17 while noble gas (He to Rn) are in group 18.
- Generally, atomic radii decrease on moving from left to right in a period, hence atomic radii of noble gases are expected to be smaller than those of halogens.
- Due to the crowding of eight electrons in the valence shell, electron density increases and there is an appreciable electronic repulsion between them.
- To decrease this repulsion and electronic density, the volume of valence shell increases.
Therefore atomic radii of noble gases are larger than those of halogens.
Question 31.
Which element has higher electron affinity among O and S? Why?
Answer:
- Oxygen has less negative electron gain enthalpy, due to high electronegativity, low atomic size and high electron density so that the incoming electron is repelled.
- Electron gain enthalpy of the elements decreases down the group due to successive decrease in electronegativity and nuclear attraction and increase in atomic size.
Element S Se Te Po O Electron gain enthalpy kJ/mol -200 -195 – 190 – 174 – 141
Question 32.
Why is the first ionisation energy of oxygen lower than that of nitrogen?
Answer:
- Electronic configuration :
7N Is2 2s2 2px1 2py1 2pz1
8O Is2 Is2 2px2 2py1 2pz1
- Nitrogen has extra stability due to half-filled p-orbitals. Therefore it has higher first ionisation enthalpy.
- Oxygen by losing one electron from 2px orbital acquires extra stability of half-filled 7-orbitals and hence has lower ionisation enthalpy.
- Therefore oxygen has lower ionisation enthalpy than nitrogen.
Question 33.
Why do halogens possess very high values of electronegativity? Arrange the halogens in the order of decreasing electronegativity.
Answer:
- Since halogens have eletronic configuration, ns2 np5, they have tendency to accept one electron to complete an octet.
- Group 17 elements have the lowest atomic radii and high effective nuclear charge.
- Therefore halogens have high values of electronegativity.
- The decreasing order of electronegativity is, F > Cl > Br > I
Question 34.
Discuss the physical states and the types of elements of Groups 16,17 and 18.
Answer:
Physical states :
- Oxygen is a gas, while the other elements of Group 16 are solids at room temperature.
- All the elements of group 16 show allotropy and exist in several allotropic modifications.
- In group 17, fluorine and chlorine are gases, bromine is a liquid and iodine is a solid at room temperature.
- All halogens are coloured. Fluorine is a yellow coloured gas, chlorine is a greenish-yellow coloured gas, bromine is a red coloured liquid and iodine is a violet coloured solid.
- All the elements of group 18 are monoatomic gases.
![]()
Types of elements :
- In group 16 elements, oxygen and sulphur are non-metals, selenium and tellurium are metalloids, while polonium is a radioactive metal with half-life 138 days.
- All halogens are non-metals.
- Group 18 elements are chemically inert and hence called inert elements.
Question 35.
Explain the colour of halogens.
Answer:
- All halogens are coloured.
| Halogen | Fluorine | Chlorine | Bromine | Iodine | Astatine |
| colour | pale yellow | greenish yellow | red | violet | metallic grey |
- The colour arises due to absorption of radiation in visible region and exCltation of electrons.
- From F to I atomic size increases, hence the valence electrons are loosely bound.
- For example, fluorine absorbs violet radiation of higher energy and transmits yellow light of lower energy while iodine absorbs yellow light of lower energy and transmits violet radiation of higher energy.
Question 36.
Explain the trend in the density, melting and boiling points of Group 16 elements.
Answer:
Density :
- The density of group 16 elements increases down the group.
- On moving down the group, the increase in atomic mass is more than the increase in atomic size.
- Down the group the magnitude of van der Waals forces of attraction increases resulting in compact lattice formation of elements.
Therefore the density increases down the group.
The density increases in the order O < S < Se < Te
Melting and boiling point :
- The melting and boiling points of the elements increase regularly on moving down the group.
- However, the melting and boiling points of polonium are lower than that of tellurium.
- This is because the intermolecular van der Waals forces are weaker in polonium.
- There is also a large difference in the melting and boiling points of oxygen and sulphur as oxygen has a smaller size, while sulphur has a larger size and stronger van der Waals forces.
| Element | 0 | S | Se | Te | Po |
| Melting point (K) | 55 | 393 | 490 | 725 | 520 |
| Boiling point (K) | 90 | 718 | 958 | 1260 | 1235 |
Question 37.
Explain the following in the case of group 17 elements :
(i) Density
(ii) Melting and boiling point
Answer:
Density :
- Down the group, density of halogens increases.
- This is, because down the group, van der Waals forces of intermolecular attraction increase, and hence tendency for agglomerisation increases. Therefore density increases.
Melting point and boiling point :
- Halogens have low melting points and boiling points.
- Melting points and boiling points increase down the group.
Question 38.
In case of group 18 elements, explain the variation in melting and boiling points.
Answer:
Melting points and boiling points :
- Group 18 elements have very low melting points and boiling points.
- The melting points and boiling points increase down the group from He to Rn.
- As the size of the atoms increases on moving down the group, the magnitude of the van der Waals forces increase from He to Rn.
- Thus, the melting and boiling points increase from He to Rn. Helium has the lowest boiling point (4.2 K) of any known substance.
- The boiling points increase in the order He < Ne < Ar < Kr < Xe < Rn
![]()
Question 39.
Explain reactions of halogens with water.
Answer:
- Halogens react with water forming halo acids and haloxyaClds.
- The reactivity decreases from fluorine to iodine.
- 2F2 + 2H2O → 4HF + O2
3F2 + 3H2O → 6HF + O3
X2(g) + H2O(1) → HX(aq) + HOX (X = Cl, Br)
Question 40.
How do halogens react with other solvents and with hydrocarbons?
Answer:
- Halogens are soluble in various organic solvents such as chloroform, carbon disulphide and carbon tetrachloride
- All halogens react with hydrocarbons and form mono or multisubstituted compounds.
- The reactivity decreases down the group from fluorine to iodine.
CH4 + 2F2 → C + 4HF
C + 2F2 → CF4
CH4 + Cl2 → CCl4 + 4HCl
Question 41.
Explain the trend in the bond dissociation enthalpies of halogen molecules.
Answer:
- The bond dissociation enthalpies of halogen molecules decrease down the group with an increase in atomic size.
- However, the bond dissociation enthalpy of fluorine is lower than that of chlorine and bromine because the F – F bond is weak.
- The bond dissociation enthalpies of the halogen molecules decreases in the order,
Cl – Cl > Br – Br > F – F > I -1.
Question 42.
Why does the density of halogens increase on moving down the group?
Answer:
The bond dissociation enthalpies of halogen molecules decrease down the group with an increase in atomic size.
Question 43.
Why do melting and boiling points of halogens increase on moving down the group?
Answer:
- The atomic size of halogens increases on moving down the group and hence the strength of van der Waals forces between the molecules also increases.
- In the lighter elements, F and Cl, these forces are weak, hence they are gases at room temperature and possess low melting and boiling points.
- In the heavier elements of the group, the van der Waals forces become stronger. Thus, bromine is a liquid and iodine is a solid and they possess higher melting and boiling points.
Question 44.
Why does oxygen show anomalous behaviour?
OR
Oxygen differs from the rest of the members of the family. Explain
Answer:
Oxygen is the first element of group 16.
Reasons for anomalous behaviour of oxygen :
- It has the smallest size in the group.
- It has the highest electronegativity (3.5).
- It does not have vacant d-orbitals like other elements in group 16.
![]()
Question 45.
Explain the anomalous behaviour of oxygen in relation to the properties of the Group 16 elements.
Answer:
Oxygen shows following anomalous behaviour :
- Physical state : Oxygen is a gas while other elements in the group are solids at ordinary temperature.
- AtomiClty : Oxygen exists as a diatomic molecule O2 while other elements are polyatomic molecules like S8, Se8 have puckered ring structure.
- Magnetic behaviour : Molecular oxygen O2 is paramagnetic while other elements are diamagnetic. Molecular O2 has two unpaired electrons in the antibonding molecular orbitals.
- Oxidation states : Oxygen shows oxidation state-2 in oxides, – 1 in peroxides while + 2 in oxygen difluoride, OF2.
- Since it does not have vacant d-orbital it doesn’t show higher oxidation states while other elements of group 16 show + 2, + 4 and + 6 oxidation states.
- Hydrogen bonding : Since oxygen has high elec-tronegativity (3.5), it forms hydrogen bonding in its compounds like H2O, alcohols, etc. Other elements in the group do not show this property.
- Hydrides : The hydride of oxygen, H2O is a liquid while the hydrides of all other elements in group 16 are gases.
- Covalency : Oxygen shows a common covalency 2 since it has only two unpaired electrons and no d -orbitals in its valence shell. In rare cases, it shows covalency 4.
- The other members of Group 16 can show covalency, more than 4, due to the presence of J-orbitals in their valence shell.
Question 46.
Oxygen generally exhibits an oxidation state of – 2, whereas the other members of its family show oxidation states of +2, +4 and +6 as well. Explain.
Answer:
- The electronic configuration of oxygen is ls22s22p4.
- It has two half filled p-orbitals and no d-orbitals for exCltation of electrons. Hence it cannot show higher oxidation states.
- Oxygen being highly electronegative, it mostly shows an oxidation state of – 2 only.
- Other members of the family like sulphur, have vacant d-orbitals, thereby giving four and six half- filled orbitals for bonding.
- Furthermore, they can combine with more electronegative elements.
- Hence, they show oxidation states of +2, +4 and 4- 6 also.
Question 47.
Why is H2O a liquid and H2S a gas?
OR
Water is a liquid and other hydrides of the group, are gases. Explain.
Answer:
- The boiling point of water (373 K) is very high hence it is less volatile while that of H2S is low (213 K) and hence it is volatile.
- Since oxygen is more electronegative than sulphur, O-H bond is more polar and there arises associ-
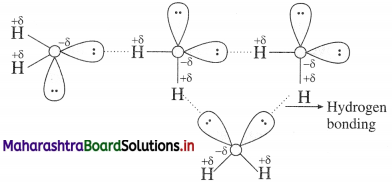
- Hydrogen bonding and molecular assoClation are not present in H2S and other hydrides.
- In H2S, there are weak van der Waals forces.
- Therefore to separate H2O molecules in H2O liquid, energy required is higher than that required to separate H2S molecules.
Hence H2O is a liquid and H2S (and other hydrides) is a gas.
Question 48.
Why does fluorine show anomalous behaviour?
Answer:
Fluorine exhibits anomalous behaviour as compared to other halogens in the group.
The reasons for anomalous behaviour of fluorine are as follows :
- the smallest size of fluorine
- the highest electronegativity
- low bond dissoClation enthalpy of F-F bond
- non-availability of d-orbitals in its valence shell.
![]()
Question 49.
Explain the anomalous properties of fluorine.
Answer:
The anomalous properties of fluorine are as follows :
- Fluorine has the highest reactivity among other halogens.
- Fluorine forms strong hydrogen bonding in its hydrides unlike other halogens.
- HF is a liquid while other hydrogen halides are gases at room temperature.
- HF is a weak aCld while other haloaClds are strong aClds.
- Fluorine shows only one oxidation state – 1 while all other halogens show variable oxidation states like -1, +1, +3, +5 and + 7.
- Fluorine has the highest electronegativity but less negative electron gain enthalpy than chlorine.
- The compounds of fluorine have higher ionic character than other halogens.
- Fluorine has no tendency to form polyhalide ion whereas other halogens form polyhalide ions like, Cl3–, Br3– and I3–.
- Fluorine unlike other halogens when reacts with water and produces O2 and O3.
2F2 + 2H2O → 4HF + O2;
3F2 + 3H2O → 6HF + O3 - Fluorine shows much higher values of ionisation enthalpy, electronegativity and standard electrode potentials compared to the other halogens.
- Fluorine shows much lower values of ionic and covalent radii, melting and boiling points and electron gain enthalpy than expected.
- Fluorine forms only one oxoacid HOF, while the other halogens form a number of oxoacids.
Question 50.
Describe anomalous behaviour of fluorine with the other elements of group 17 with reference to :
(a) Hydrogen bonding
(b) Oxidation state
(c) Polyhalide ions.
Answer:
Anomalous behaviour of fluorine with other 17 group elements (Cl, Br, I) :
(a) Hydrogen bonding : Hydrogen bonding is present in HF only, while it is absent in other haloaClds (HCl, HBr and HI).
(b) Oxidation state : Fluorine shows only one oxidation state namely – 1 due to absence of d-orbital. Other halogens exhibit along with – 1 other oxidation states namely +1, +3, + 5 and +7.
(c) Polyhalide ion : Fluorine does not form polyhalide ion but other halogens form polyhalide ions like Cl–3, Br–3 and I–3.
Question 51.
At room temperature hydrogen fluoride is a liquid while all other hydrogen halides are gases. Explain.
Answer:
- Fluorine has the highest electronegativity compared to remaining halogens.
- H-F bond has the highest bond polarity compared to other halides.
- The presence of strong intermolecular hydrogen bonding leads to the association of HF molecules as follows :
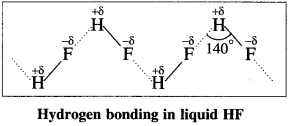
- Other hydrogen halides do not show hydrogen bonding due to their larger atomic size and less electronegativity.
Hence HF is a liquid while other hydrogen halides (HCl to HI) are gases.
Question 52.
Explain the oxidation states of Group 16 elements.
Answer:
- Group 16 elements have general electronic configuration, ns2 np4,
- They show variable oxidation states, – 2, + 2, + 4 and -6.
- Since all elements have 6 valence electrons, they tend to gain or share 2 electrons to complete an octet, and show common oxidation state – 2.
- Oxygen being highly electronegative, it shows the common oxidation state -2 in oxides (H2O, MgO). It shows – 1 oxidation state in peroxides (H2O2, Na2O2) and + 2 oxidation states in OF2.
- Except oxygen all other elements have vacant d-orbitals, hence they show higher oxidation states + 4 and + 6. For example

- The stability of the + 6 oxidation state decreases but the stability of the +4 oxidation state increases down the group due to the inert pair effect.
- Bonding in +4 and +6 oxidation states is covalent in nature.
![]()
Question 53.
What is the oxidation state of S in H2S2O6?
Answer:
![]()
∴ The oxidation state of S atom = + 5
Question 54.
What is the oxidation state of oxygen in OF2?
Answer:
The oxidation state of oxygen in OF2 is +2.
![]()
Question 55.
What is the oxidation state of oxygen in compounds
(i) O2F2 and
(ii) H2O2?
Answer:
(i) In O2F2, the oxidation state of oxygen is + 1.
![]()
(ii) In H2O2, the oxidation state of oxygen is – 1.
![]()
Question 56.
Oxygen usually exhibits – 2 oxidation state, but it exhibits + 2 oxidation state in OF2?
Answer:
- Oxygen being highly electronegative, it shows the common oxidation state – 2 in oxides.
- In the presence of a highly electronegative fluorine atom it exhibits + 2 oxidation state in OF2.
Question 57.
Why do sulphur and other heavier elements of group 16 exhibit higher oxidation states?
Answer:
Since sulphur and heavier elements of group 16 have large atomic radii and available d-orbitals, they exhibit higher oxidation states.
Question 58.
Why does the tendency of group 16 elements to exist in -2 oxidation state decrease on moving down the group?
Answer:
- The electronegativity of the elements of Group 16 elements decreases on moving down the group in the order O > S > Se > Te > Po.
- Thus, the tendency to show -2 oxidation state also decreases.
Question 59.
Discuss the oxidation states in Group 18 eiements.
Answer:
- The group 18 elements have a stable electronic configuration ns2np6 with completely filled orbitals.
- Due to completely filled orbitals and complete octets these elements do not show a tendency to lose, gain or share electrons.
- They have zero valency and mostly exist as mono- atomic gases.
- Xenon exhibits higher oxidation states, as the paired electrons of the valence shell can be unpaired and promoted to the empty d-orbitals.
- The unpaired electrons are shared with fluorine or oxygen atoms to form covalent compounds with higher oxidation states such as XeF2, XeF4, XeF6, XeO3 and XeOF6.
Question 60.
Why does xenon being a noble gas form compounds with other elements?
Answer:
Xenon forms compounds with other elements due to the following reasons :
- Xenon has large atomic size and lower ionisation enthalpy.
- The paired electrons of the valence shell can be unpaired and promoted to the empty d-orbitals.
- The unpaired electrons are shared with fluorine or oxygen atoms to form covalent compounds with higher oxidation states.
![]()
Question 61.
Explain the reactivity of group 16 elements with hydrogen.
Answer:
- All the elements of group 16 react with hydrogen and form hydrides of the type H2M where M = O, S, Se, Te and Po. For example H2O, H2S, H2Se, H2Te and H2Po.
- The hydrides of these elements show regular trends in their physical and chemical properties.
Question 62.
Explain the general structures of hydrides of group 16 elements.
OR
Give reason : Bond angle decreases from H2O to H2S.
Answer:
- Group 16 elements form hydrides of the type H2M where M = O, S, Se, Te and Po.
- All these hydrides are formed by sp3 hybridisation of the central atom and have angular shape.
- All hydrides have similar structure but differ in H-M-H bond angle. This bond angle decreases from H2O to H2Te.
- All these hydrides have two M-H bond pairs and two lone pairs of electrons.
- Due to repulsion between lone pairs-lone pairs and lone pairs-bond pairs, and bond pairs-bond pairs the bond angle is reduced from regular 109° 28′.
- On moving down the group, atomic size increases, electronegativity decreases, electron density decreases.
- Since among group 16 elements, oxygen has the highest electronegativity, lowest atomic size and hence the highest electron density, the repulsion between electron pairs is maximum in H2O, hence H-O-H bond angle is maximum.
- The electron pair repulsion decreases down the group, hence bond angle also decreases down the group.
| Hydrides | H2O | H2s | H2Se | H2Te |
| H-M-H angle | 104° | 92° | 91° | 90° |
Question 63.
Explain the physical states, volatility and chemical properties of hydrides of group 16 elements.
Answer:
(1) Physical state : Hydride of oxygen, H2O is a colourless odourless liquid while the hydrides of other group 16 elements are colourless poisonous gases with unpleasant odours.
(2) Volatility : Volatility of hydrides increase from H2O to H2S and then decreases. H2O < H2S > H2Se > H2Te
At ordinary temperature H2O is a liquid while all other hydrides are gases.
(3) Thermal stability :
- The thermal stability of hydrides decreases in the order of H2O > H2S > H2Se > H2Te.
- Since atomic size increases from O to Te, the tendency to form hydride bond, M-H decreases. Hence M-H bond in O-H is the strongest and in Te-H the weakest. Therefore thermal stability decreases from H2O to H2Te.
(4) ACldic character :
- The hydrides of group 16 elements are weakly acidic and acidic strength increases from H2O to H2Te.
Since M-H bond strength decreases, bond enthalpy decreases, acidic character increases.
(5) Reducing power :
- Except H2O, all hydrides of group 16 elements are reducing agents.
- Reducing power increases from H2S to H2Te.
- Reducing power of the hydrides is due to their less stability and tendency to dissociate, which increases from H2S to H2Te.
Question 64.
Giving suitable reasons, arrange the hydrides of group 16 elements in the decreasing order of their thermal stability.
Answer:
- The thermal stability of hydrides decreases in the order of H2O > H2S > H2Se > H2Te.
- Since atomic size increases from O to Te, the tendency to form hydride bond, M-H decreases.
- Hence M-H bond in O-H is the strongest and in Te-H the weakest. Therefore thermal stability decreases from H2O to H2Te.
![]()
Question 65.
What is the oxidation state of S in the following :
(a) S8
(b) \(\mathrm{HSO}_{4}^{-}\)
(c) K2S2Og?
Answer:
(a) Oxidation state of S in, S8 is zero.
(b) In \(\mathrm{HSO}_{4}^{-}\) it is +6
(c) in K2S2O8 it is +6
Question 66.
H2S is less acidic than H2Te, why?
Answer:
- Sulphur is more electronegative than Tellurium.
- Bond energy of S-H in H2S is (347 kJ mol-1) more than the bond energy of Te-H (238 kJ mol-1).
- Hence H2Te dissociates more giving H + (or H3O+) than H2S in the solution. Therefore H2S is less acidic than H2Te.
Question 67.
“The reducing power of the hydrides of group 16 elements increases from H2S to H2Te”. Explain.
Answer:
- Except H2O, all hydrides of group 16 elements are reducing agents.
- reducing power increases from H2S to H2Te.
- reducing power of the hydrides is due to their less stability and tendency to dissociate, which increases from H2S to H2Te.
Question 68.
Explain the volatility of hydrides from H2S to H2Te.
OR
“The boiling points of the hydrides of Group 16 elements increases from H2S to H2Te.” Explain.
Answer:
- In group 16 elements, except H2O which is a liquid, all other hydrides are gases.
- From H2S to H2Te boiling point increases due to an increase in atomic size from S to Te.
- The magnitude of van der Waals forces increases as atomic size increases, from S to Te, therefore boiling points increase from H2S to H2Te.
| Hydrides | H2o | H2S | H2Se | H2Te |
| Boiling point K | 373 | 213 | 232 | 269 |
Question 69.
Give reasons : H2S has a lower boiling point than H2O.
Answer:
- Hydrogen bonding and molecular association are not present in H2S.
- The H2O molecules are associated with each other through intermolecular hydrogen bonding.
- Hence, H2S has a lower boiling point than H2O.
Question 70.
Among the hydrides of group 16, water shows unusual properties. Why?
Answer:
- Oxygen being more electronegative, the O-H bond is more polar and there arises association of H2O molecules through intermolecular hydrogen bonding.
- The other hydrides of group 16 do not form H bonds and hence exist as discrete molecules.
- As a result, water shows unusual properties like high B.P, high thermal stability and weaker acidic character as compared to the other hydrides of group 16.
![]()
Question 71.
Explain the reactivity of halogens towards hydrogen.
Answer:
- All halogens react with hydrogen and form hydrogen halides HX where X = F, Cl, Br, I.
- The affinity and reactivity decrease down the group from F to I.
Question 72.
Explain the acidic strength of halo acids.
OR
Write a short note on the acidic strength of hydrogen halides.
Answer:
- The aCldic strength of haloaClds vary in the following order, HF < HCl < BHr < HI
- The stability of hydrogen halides decreases down the group. HF > HCl > HBr > HI.
This is because from F to I, atomic size increases and bond dissociation enthalpy of H-X decreases.
Question 73.
HF is the most stable among all the hydrogen halides. Explain.
OR
Explain the thermal stability of hydrogen halides.
Answer:
- HF is the most stable among all the hydrogen halides.
- The thermal stability decreases from HF to HI.
- This is due to a decrease in bond dissociation enthalpy and bond strength of H-X bond from F to I.
- Down the group, from F to I, atomic size increases, bond length of H-X bond increases, bond polarity decreases and hence bond strength decreases.
- F being of the lowest atomic size and the highest electronegativity, HF bond is stronger and highly thermally stable.
Question 74.
Explain the reducing character of hydrogen halides.
Answer:
- The reducing character of hydrogen halides increases from HF to HI.
- HF does not show any reducing property while HI is a strong reducing agent.
- H-X bond strength and thermal stability of hydrogen halides decrease from HF to HI.
Unlike other hydrogen halides, HF does not dissociate releasing hydrogen, hence HF is not a reducing agent.
Question 75.
Why is HCl a weaker acid than HI?
Answer:
- Bond length of HCl is less than HI.
- Cl is more electronegative than I.
- Hence bond strength of HCl is more than HI.
- Therefore HCl dissociates less than HI, which makes HCl a weaker acid than HI.
Question 76.
Why is HF a weaker acid than HCl2
Answer:
- The H-F bond is stronger than H-Cl bond.
- Hence, HF ionises less readily than HCl in H2O, to give H+ ions.
Therefore, HF is a weaker acid than HCl.
![]()
Question 77.
Arrange the halogen acids in the decreasing order of :
(1) thermal stability
(2) acidic strength.
Answer:
(1) Thermal stability : As the atomic size increases from F to I, the bond dissociation enthalpy of H-X decreases.
Thus, the thermal stability of hydrogen halides decreases down the group in the order HF > HCl > HBr > HI
(2) ACldic strength : Since, the bond dissociation enthalpy of H-X decreases down the group, the acidic strength varies in the order HI > HBr > HCl > HF
Question 78.
Noble gases do not form compounds with hydrogen. Why?
Answer:
Noble gases have a stable electronic configuration, and so are unreactive. Since, they are chemically inert, they do not form compounds with hydrogen.
Question 79.
Explain the reactivity of group 16 elements with oxygen.
OR
Give brief account of the oxides of group 16 elements.
Answer:
- All elements of group 16 react with oxygen and form oxides of the types EO2 and EO3 where E = S, Se, Te or Po. Examples are SO2, SeO2, TeO2, SO3, TeO3, etc.
- SO2 and SeO2 are acidic in nature and react with water to form acids.

- The reducing power reduces from SO2 to TeO2. SO2 is an oxidising agent, while TeO2 is a reducing agent.
- SO3, SeO3 and TeO3 are also acidic in nature. They react with water to form acids.

Question 80.
Complete the following reactions :
(i) S + O2(air)
(ii) Se + O2(air)
Answer:
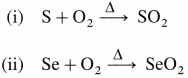
Question 81.
Explain the reactivity of halogens towards oxygen.
Answer:
- Halogens with oxygen form many oxides with different oxidation states of halogens.
- Fluorine forms O2F2 and OF2.
- Chlorine forms oxides like
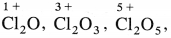
 All oxides are oxidising agents.
All oxides are oxidising agents. - Bromine forms oxides like Br2O, BrO2, BrO3 and iodine forms I2O4, I2O5, I2O7.
Question 82.
Discuss the properties of the oxides of halogens.
Answer:
Properties of oxides of halogens :
- Both OF2 and O2F2 are strong fluorinating agents. Only OF2 is thermally stable at 298 K. OF2 oxidises plutonium to PuF6. This reaction is used to remove Pu as PUF6 from spent nuclear fuel.
- The chlorine oxides Cl2O, ClO2, Cl2O6 and Cl2O7 are highly reactive oxidizing agents and tend to explode. ClO2 is used as a bleaching agent in the paper industry and textiles and in water treatment.
- Bromine forms oxides like Br2O, BrO2, BrO3, which are the least stable halogen oxides. They are powerful oxidising agents.
- Iodine forms oxides like I2O4, I2O5 and I2O7. These solids are insoluble in water and decompose on heating. I2O5 is a powerful oxidising agent and is used to determine the amount of carbon monoxide.
For a particular halogen, higher oxides are more stable than the lower ones.
![]()
Question 83.
Explain the reactivity of group 16 elements towards halogens.
Answer:
- The group 16 elements with halogens form a large number of halides of the type EX2, EX4 and EX6 where E is an element of the group and X is a halogen.
- The stability of the halides decreases in the order fluoride > chloride > bromide > iodide.
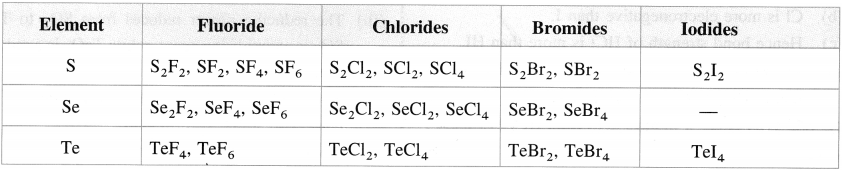
- The hexahalides SF6, SeF6, TeF6 are formed by direct combination. They are colourless gases. The hexahalides have sp3d2 hybridisation and have an octahedral structure. SF6 is exceptionally stable due to steric reasons.
- The tetrahalides SF4, SeF4, TeF4, TeCl4 have sp3d hybridisation and thus have a trigonal bipyramidal structure in which one of the equatorial positions is occupied by a lone pair of electrons. This geometry is also called see-saw geometry.
- The dihalides SCl2, SeCl2, TeCl2 have sp3 and thus have a tetrahedral structures with two equatorial positions occupied by lone pairs.
- The monohalides S2F2, S2Cl2, Se2Cl2 and Se2Br2 are dimeric in nature. These halides have a tendency to undergo disproportionation. For example, 2Se2Cl2 → SeCl4 + 3Se
Question 84.
Complete the following reaction :
(i) S + 3F2
(ii) Se + 2Cl2
Answer:
(i) S + 3F2 → SF6
(ii) Se + 2Cl2 → SeCl4
Question 85.
Explain the reactivity of halogens towards other halogens.
OR
What are interhalogen compounds?
Answer:
- The halogens have a tendency to combine amongst themselves forming different compounds called interhalogen compounds.
- Interhalogens are of the type XX, XX’3, XX’5 and XX’7 where X is larger size halogen than X’.
- They are covalent, diamagnetic, reactive and good oxidising agents.
- Preparation :
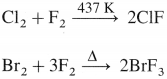
Question 86.
Explain the reactivity of Group 18 towards halogens.
Answer:
- Group 18 elements are chemically inert.
- However, inert elements like krypton and xenon react directly with fluorine under appropriate conditions to give their fluorides. For example,
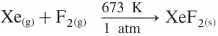
- The xenon fluorides XeF2, XeF4 and XeF6 are colourless crystalline solids which sublime at 298 K.
- These fluorides are strong fluorinating agents.
Question 87.
What is the action of metals on group 16 elements?
Answer:
Metals react with O, S, Se to form oxides, sulphides and selenides respectively.

![]()
Question 88.
Complete the following reactions :
(i) Cu + S
(ii) Cd + Se
Answer:
(i) Cu + S → CuS
(ii) Cd + Se → CdSe
Question 89.
Explain the reactivity of halogens with metals.
Answer:
- All halogens react with metals instantly to give metal halides.
- Down the group reactivity decreases from fluorine to iodine.
- 2Na(s) + Cl2(1) → 2NaCl(s), Mg(s) + Br2(s) → MgBr2(s)
- Halogens being highly electronegative, the metal-halogen bonds are ionic and ionic character decreases down the group. For example M-F > M-Cl > M-Br > M-I.
- The metal halides with higher oxidation state of the metal are more covalent than the halides with lower oxidation state of metal. For example SnCl4, PbCl4, SbCl5 and UF6 are more covalent than
Question 90.
Explain the property of allotropy in Group 16 elements.
Answer:
- All the elements of group 16 exhibit allotropy.
- These elements exist in different allotropic modifications.
- Oxygen exists as O2 and ozone O3.
- Sulphur exists as α-sulphur, β-sulphur, γ-sulphur, homocyclic sulphur, plastic sulphur, etc. Rhombic sulphur (α sulphur) and mono-clinic sulphur (β sulphur) are the important allotropes. Both are non-metallic in nature.
- Selenium exists in two allotropic forms red (non-metallic) and grey (metallic).
- Tellurium exists in two allotropic forms crystalline form and the amorphous form.
- Po has two forms namely α-form and β-form both being metallic.
Question 91.
What are the allotropes of oxygen?
Answer:
Allotropes of oxygen :
- Oxygen O2
- Ozone O3
Question 92.
Which are the most important allotropic forms of sulphur?
Answer:
The most important allotropes of sulphur are :
- Rhombic (α-sulphur)
- Monoclinic (β-sulphur)
Question 93.
Write a note on the following :
(1) Rhombic sulphur
(2) Monoclinic sulphur
Answer:
(1) Rhombic sulphur :
- Rhombic sulphur are orthorhombic crystals.
- This is the most stable form and common form of sulphur.
- It is pale yellow., having density 2.069 g/cm3 and melting point 385.8 K
- It is insoluble in water, but soluble in CS2
- It is stable below 369 K and transforms to β-sulphur above this temperature.
- It exists as S8 molecules with a structure of a puckered ring.
- It is obtained by the evaporation of roll sulphur is CS2.

(2) Monoclinic sulphur :
- Monoclinic sulphur (β sulphur or prismatic sulphur) are needle-shaped monoclinic crystals.
- It is bright yellow, having a density 1.989 gcm3 and melting point 393 K.
- It is soluble in CS2.
- It is stable above 369 K and transforms into α-sulphur below this temperature.
- It exists as, S8 molecules with a structure of a puckered ring.
- It is prepared by melting rhombic sulphur and cooling it till a crust is formed. Two holes are pierced in the crust and the remaining liquid is poured to obtain needle-shaped crystals of monoclinic sulphur.
![]()
Question 94.
What are the different oxyacids of sulphur?
Answer:
Sulphurous acid : H2SO3
Sulphuric acid (Oil of vitriol): H2SO4
Di or pyrosulphuric acid or oleum H2S2O7
Peroxy monosulphuric acid or Caro’s acid H2SO5
Peroxy sulphuric acid (or Marshall’s acid : H2S2O8
Thiosulphuric acid H2S2O3
Question 95.
Write the structures of oxyacids of sulphur. Write the oxidation number of sulphur.
Answer:
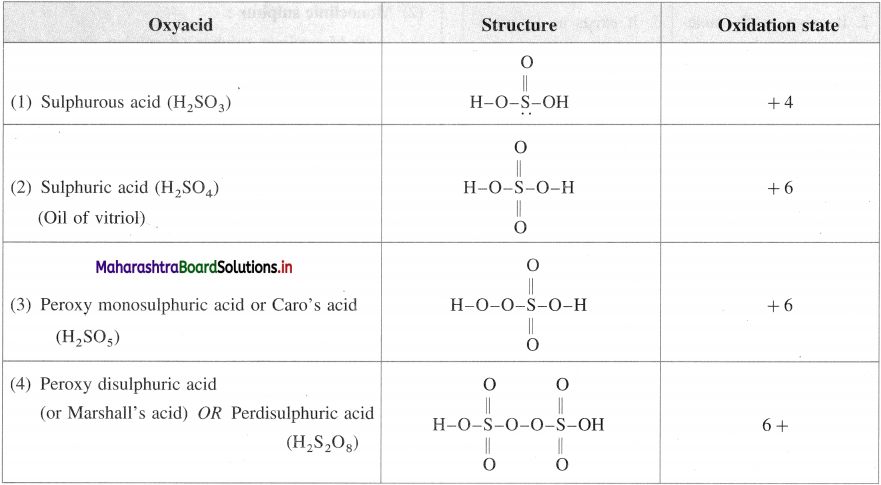

Question 95.
Write molecular formulae and structure of the following compounds :
(1) Peroxy monosulphuric acid
(2) Pyrosulphuric aCld
Answer:
(1)
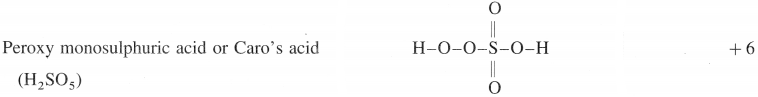
(2)
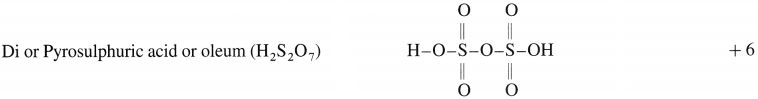
![]()
Question 96.
What is oil of vitriol?
Answer:
In ancient days, sulphuric acid, H2SO4 was called, oil of vitriol.
Question 97.
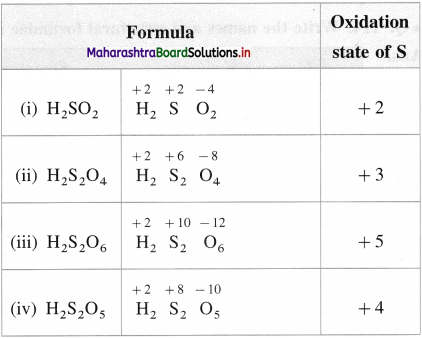
What is the oxidation state of S in the following compounds?
(i) H2SO2
(ii) H2S2O4
(iii) H2S2O6
(iv) H2S2O5
Answer:
Question 98.
Which are different oxyacids (or oxoacids) of halogens?
Answer:
Fluorine forms only one oxyacid namely, hypofluorous acid or fluoric acid, HOF while other halogens form several oxyacids.

Question 99.
How do
(i) oxidising power and
(ii) thermal stability of oxyacids (or oxoacids) of halogens vary?
Answer:
(i) Oxidising power of oxyacids of halogens decreases as the oxidation number of halogens increases.

(ii) The thermal stability of oxyacids of halogens increases with the increase in the oxidation state of halogen. Hence the increasing order of thermal stability is,
![]()
Question 100.
How does the acid strength of the halogen oxoacids vary?
Answer:
The acid strength of the halogen oxoacids increases with increase in the oxidation state of the halogen.
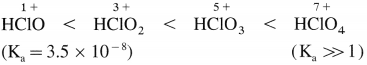
![]()
Question 101.
Explain the laboratory methods for the preparation of dioxygen.
Answer:
Laboratory methods :
(i) By heating chlorates, nitrates and permanganates.
Potassium chlorate in the presence of manganese dioxide on heating decomposes to form potassium chloride and oxygen.
![]()
(ii) By the thermal decomposition of metal oxides.
![]()
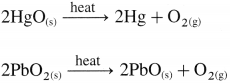
(iii) By the thermal decomposition of hydrogen peroxide :
The thermal decomposition of hydrogen peroxide in the presence of finely divided metal and manganese dioxide used as catalyst gives oxygen.
![]()
Question 102.
How is oxygen prepared by thermal decomposition of certain metallic oxides?
Answer:

Question 103.
Write the reactions for the decomposition of following oxides on heating :
(i) HgO
(ii) Ag2O
(iii) PbO2
(iv) H2O2.
Answer:

Question 104.
How is dioxygen manufactured on a large scale?
Answer:
Dioxygen on a large scale or commercial scale is obtained by two following methods :
(1) From water : By electrolysis of acidified water, H2 gas is obtained at the cathode and O2 is obtained at anode.
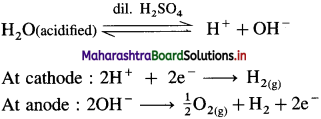
(2) From air :
- Carbon dioxide and water vapour is removed from air and the remaining gases are liquefied.
- O2 on large scale is obtained by fractional distillation of liquid air.
- On distillation, liquid dinitrogen having low boiling point distils out first leaving behind liquid dioxygen. Then liquid O2 is distilled out and separated.
![]()
Question 105.
What is the action of heat on KClO3?
Answer:
Laboratory methods :
(i) By heating chlorates, nitrates and permanganates.
Potassium chlorate in the presence of manganese dioxide on heating decomposes to form potassium chloride and oxygen.
\(2 \mathrm{KClO}_{3} \frac{\Delta}{\mathrm{MnO}_{2}} 2 \mathrm{KCl}+3 \mathrm{O}_{2(\mathrm{~g})}\)
(ii) By the thermal decomposition of metal oxides.
\(2 \mathrm{Ag}_{2} \mathrm{O}_{(\mathrm{s})} \stackrel{\text { heat }}{\longrightarrow} 4 \mathrm{Ag}+\mathrm{O}_{2(\mathrm{~g})}\)
\(\begin{aligned}
&2 \mathrm{HgO}_{(\mathrm{s})} \stackrel{\text { heat }}{\longrightarrow} 2 \mathrm{Hg}+\mathrm{O}_{2(\mathrm{~g})} \\
&2 \mathrm{PbO}_{2(\mathrm{~s})} \stackrel{\text { heat }}{\longrightarrow} 2 \mathrm{PbO}_{(\mathrm{s})}+\mathrm{O}_{2(\mathrm{~g})}
\end{aligned}\)
(iii) By the thermal decomposition of hydrogen peroxide :
The thermal decomposition of hydrogen peroxide in the presence of finely divided metal and manganese dioxide used as catalyst gives oxygen.
\(2 \mathrm{H}_{2} \mathrm{O}_{2 \text { (aq) }} \frac{\text { heat }}{\mathrm{MnO}_{2}} 2 \mathrm{H}_{2} \mathrm{O}_{(1)}+\mathrm{O}_{2(\mathrm{~g})}\)
Question 106.
Write the reactions of dioxygen with
(i) ZnS
(ii) CH4
Answer:
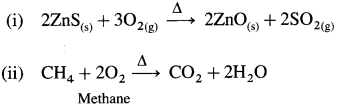
Question 107.
Complete the following reactions :

Answer:

Question 108.
What are the uses of dioxygen?
Answer:
The uses of dioxygen are as follows :
- Dioxygen is essential for sustaining life. It is used in hospitals for artificial respiration.
- It is used in oxy-hydrogen (2800 °C) and oxy-acetylene (3200 °C) torches used for cutting and welding metals.
- Oxygen cylinders are used in hospitals, for high altitude flying and in mountaineering.
- It is used in the combustion of fuels. In rockets, hydrazine in oxygen is used as a fuel as it provides tremendous thrust.
Question 109.
What are oxides? How are they classified? Give examples.
OR
What are the different types of oxides? Give example.
Answer:
The oxides are binary compounds in which one element is oxygen and another may be a metal or a non-metal.
They are classified as follows :
- acidic oxides, CO2, SO2, etc.
- Basic oxides, CaO, BaO, etc.
- Amphoteric oxides, Al2O3, ZnO, etc.
- Neutral oxides, NO, N2O, CO, etc.
![]()
Question 110.
Explain different types of oxides.
Answer:
Oxides are of four types as follows :
(1) acidic oxides :
- The oxide, which on reaction with water forms an acid or reacts with a base to give a salt is called an acidic oxide.
- It is formed by the combination of oxygen with non-metals. For example CO2, SO3, etc.

(2) Basic oxide :
- The oxide, which on reaction with water forms a base or reacts with an acid to give a salt is called basic oxide.
- It is formed by the reaction of oxygen with highly electropositive metals.
For example, Na2O, CaO, etc.
Na2O(s) + H2O(1) → 2NaOH(aq)
CaO(s) + H2O(1) → Ca(OH)2(aq)
BaO(s) + 2HCl(aq) → BaCl2 + H2O - Basic oxides are generally ionic in nature.
(3) Amphoteric oxide :
- The oxide, which shows both acidic and basic I characteristics is called an amphoteric oxide.
- They are formed by the reaction of oxygen with elements which lie on the border of electroposi- five (metals) and electronegative (non-metals) nature.
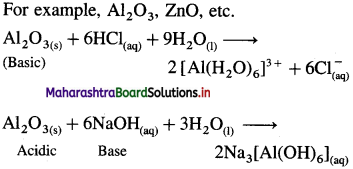
Along the period, from left to right the nature of oxides changes from basic to amphoteric to acidic.
(4) Neutral oxide : The oxide which behaves neither acidic nor basic is called a neutral oxide.
For example, CO, NO, N2O.
Question 111.
What is the nature of the following oxides :
(i) N2Os
(ii) P4O10
(iii) Cl2O7
(iv) ZnO
(v) Al2O3
(vi) K2O
(vii) BaO
(viii) CO
(ix) N2O?
Answer:
| Acidic oxide | (i) N2O5 (ii) P4O10 (iii) Cl2O7 |
| Basic oxide | (vi) K2O (vii) BaO |
| Amphoteric oxide | (iv) ZnO (v) Al2O3 |
| Neutral oxide | (viii) CO (ix) N2O |
Question 112.
Complete the following reactions :
(i) P4O10 + H2O
(ii) C12O7 + H2O
(iii) ZnO + HCl
(iv) ZnO + NaOH
(v) Al2O3 + NaOH.
Answer:

![]()
Question 113.
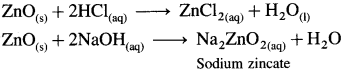
Explain the nature of zinc oxide with the help of the reactions.
OR
Explain with chemical reactions, why is zinc oxide amphoteric in nature.
Answer:
Question 114.
What is ozone umbrella?
OR
Explain Ozone as a protective umbrella for UV from sun.
Answer:
The stratospheric pool of ozone which is a layer above earth’s surface and protects from harmful high energetic ultraviolet (UV) rays is called the ozone umbrella or ozonosphere.
Question 115.
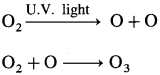
How is ozone formed naturally?
Answer:
- In the atmosphere, ozone is naturally formed through photochemical reactions.
- Oxygen present in the lower mesosphere on the absorption of solar radiations, is dissociated into two oxygen atoms which oxidise oxygen to ozone.
- One atomic oxygen combines with molecular oxygen to form O3.
Question 116.
Explain laboratory preparation of ozone.
Answer:
- When a slow dry stream of oxygen is passed through a silent electric discharge, oxygen is converted into ozone (about 10%). The mixture is called ozonised oxygen.

- It is an endothermic reaction.
- Silent electric discharge prevents the decomposition of ozone.
Question 117.
What are the physical properties of ozone?
Answer:
The physical properties of ozone are as follows :
- Gaseous ozone is blue, liquid ozone is dark blue and solid ozone is violet-black.
- It has a pungent odour hence the name is ozone.
- At higher concentrations (about 100 ppm), it is harmful and results into nausea and headache while in small concentrations it is harmless.
- Ozone is thermodynamically less stable than oxygen.
- The decomposition of ozone (2O3 → 3O2) is exothermic (ΔH < O) and has ΔS > 0.
- Therefore ΔG < 0 and hence decomposition of O3 is spontaneous.
- Ozone is diamagnetic in nature.
Question 118.
Ozone acts as an oxidising agent and a reducing agent. Explain with examples.
Answer:
(A) Ozone as an oxidising agent : Since ozone decomposes to liberate nascent oxygen, it is a powerful oxidising agent next to fluorine.
O3(g) → O2(g) + O
For example :
- It oxidises lead sulphide (PbS) to lead sulphate (PbSO4) changing the oxidation state of S from – 2 to +6.
PbS(s) + 4O3(g) → PbSO(s) + 4O2(g) - Potassium iodide, KI is oxidised to iodine, I3 in the solution.
2KI(aq) + H3O(1) + O3(g) → 2KOH(aq) + I2(s) + O2(g) - Ozone oxidises nitrogen oxide to nitrogen dioxide.
NO(g) + O3(g) → NO2(g) + O2(g)
![]()
(B) Ozone as a reducing agent : Ozone reduces peroxides (O1-) to oxides (O2-).
- Ozone reduces barium peroxide, BaO2 to barium oxide, BaO.
BaO2 + O3 → BaO + 2O2 - Ozone reduces hydrogen peroxide, H2O2 to water, H2O.
H2O2 + O3 → H2O + 2O2
Question 119.
What is meant by ozone depletion?
Answer:
The thinning of the ozone layer in the upper atmosphere is called ozone depletion. This thinning has been more pronounced in the polar regions, especially over the Antarctica.
Question 120.
How does ozone protect the people of the earth?
Answer:
Ozone layer in the upper atmosphere absorbs the harmful high energetic UV radiations of the Sun and protects the earth. The ultraviolet radiations damage plant and animal life on earth.
Question 121.
How do exhaust systems of cars and supersonic jet aeroplanes deplete the concentration of ozone layer?
Answer:
The exhaust gases from cars and supersonic jet aeroplanes contain nitric oxide (NO), which combines with ozone in the atmosphere forming oxygen.
NO + O3 → NO2 + O2
Hence the concentration of ozone is depleted in the upper layer of the atmosphere.
Question 122.
How do the coolents in refrigerants deplete the concentration of ozone?
Answer:
- The coolents used in refrigerants are generally chlorofluorocarbons, CFCs also known as Freon, for example CF2Cl2.
- CFCs have very long lifetime about 20 to 100 years.
- CF2Cl2 undergoes photochemical decomposition giving free radicals which react and destroy ozone.

Thus Cl- propagates the destruction of ozone in the atmosphere.
Question 123.
What is the action of ozone on unsaturated hydrocarbons? Give reaction.
Answer:
Ozone with unsaturated hydrocarbons form ozonides.

Question 124.
Ozone depletion is a major environmental problem. Explain.
Answer:
- The depletion of ozone layer increases the amount of ultraviolet radiation reaching the Earth.
- This has caused an increase in the rate of skin cancer, eye cataracts and damage to the genetic as well as immune system.
- Thus, ozone depletion has become a major environ-mental problem.
Question 125.
What are the uses of ozone?
Answer:
Uses of Ozone :
- Ozone sterilises drinking water by oxidising germs and bacteria present in it.
- It is used as a bleaching agent for ivory, oils, starch, wax and delicate fabrics like silk.
- Ozone is used to purify the air in crowded places like Clnema halls, railways, tunnels, etc.
- In industry, ozone is used in the manufacture of synthetic camphor, potassium permanganate, etc.
![]()
Question 126.
How is sulphur dioxide prepared?
Answer:
Sulphur dioxide, SO2 is prepared by following methods :
(1) From sulphur : When sulphur is burnt in air or oxygen, SO2 is formed along with SO3.
![]()
(2) Laboratory method (From sulphite) :
- Sodium sulphite on treating with dilute H2SO4 forms SO2.
Na2SO3 + H2SO4(aq) → Na2SO4 + H2O(1) + SO2(g) - Sodium sulphite, Na2SO3 on reaction with dilute hydrochloric acid solution forms SO2.
Na2SO3(aq) + 2HCl(aq) → 2NaCl9aq0 + H2O(1) + SO2(g)
(3) Industrial method (From sulphides): It is obtained as a by-product in the roasting of ores of pyrites and blendes.

The gas is dried and liquefied under pressure and stored.
Question 127.
What happens when an excess of SO2 is passed through sodium hydroxide solution?
Answer:
When SO2 gas is passed through sodium hydroxide solution (NaOH), it forms sodium sulphite, Na2SO3 which further with excess of SO2 forms sodium hydrogen sulphite, NaHSO3.
2NaOH + SO2 → Na2SO3 + H2O
Na2SO3 + H2O + SO2 → 2NaHSO3
Question 128.
Give reactions to show that SO2 gas acts as a reducing agent.
Answer:
Sulphur dioxide (SO2) acts as a reducing agent in the presence of moisture.
- Sulphur dioxide, SO2 reduces halogens (X°) to haloacids (X1-).
I2 + 2H2O + SO2 → 2HI + H2SO4 - SO2 gas when passed through an acidified solution of KMnO4 it is decolourised due to reduction (Mn7+ to Mn2+).
2KMnO4 + 2H2O + 5SO2 → K2SO4 + 2MnSO4 + 2H2SO4 - Sulphur dioxide (SO2) reduces Fe3+ to Fe2+
2FeCl3 + SO2 + 2H2O → 2FeCl2 + H2SO4 + 2HCl
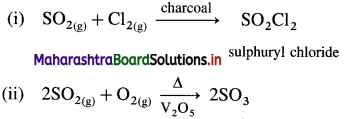
Question 129.
Complete the following reactions :

Answer:
Question 130.
What are the uses of sulphur dioxide?
Answer:
The uses of sulphur dioxide are as follows :
- SO2 is used in the manufacture of H2SO4.
- In refining petroleum and also in sugar industry.
- In the manufacture of NaHSO3.
- As an antichlor, as a disinfectant and preservative.
- Liquid SO2 is used as a selective solvent to dissolve many inorganic and organic compounds.
- Sulphur dioxide is used for bleaching wool and silk. As a bleaching agent in moist condition due to reduction reaction (SO2 + 2HaO → H2SO4 + 2[H]) colouring matter + [H] → colourless matter.
- On exposing the bleached matter, the colour is restored due to oxidation. Colourless matter + [O] → coloured matter. Hence the bleaching action is temporary.
![]()
Question 131.
Explain, the nature of S-O bond in SO2.
Answer:
- In SO2, sulphur atom is sp2 hybridised forming three hybrid orbitals.
- In SO2, each oxygen atom is bonded to sulphur by a σ and a π bond. Hence in SO2 there are two σ and two π bonds.
- a bonds are formed by sp2-p overlap while one of n bonds is due to pπ-pπ overlaps and other is due to pπ-pπ overlap.
- But both S-O bonds are identical due to resonance and has bond length 143 pm.
Question 132.
What are the physical properties of H2SO4?
Answer:
- It is a colourless, dense, oily liquid having speClfic gravity 1.84 at 298 K.
- It has freezing point 283 K and boiling point 611 K.
- It is a strong dehydrating agent and dissolves in water with the evolution of a large amount of heat.
- It is a strong dibasic acid and acts as an oxidising agent.
- Due to hydrogen bonding, it is viscous.
- It is highly corrosive and produces severe bums on skin.
Question 133.
How is a solution of H2SO4 prepared from concentrated sulphuric acid solution?
Answer:
- Concentrated H2SO4 dissolves in water with the evolution of a large amount of heat.
- Generally a dilute solution is prepared by adding water to the concentrated solution.
- In case of concentrated H2SO4, since its specific gravity is higher, the added water remains on the surface of it and due to evolution of heat, a glass vessel cracks at the interface.
- Hence concentrated H2SO4 must be added slowly into fine stream of water with constant stirring to obtain a dilute H2SO4 solution.
Question 134.
Give two chemical reactions to explain oxidizing property of concentrated H2SO4.
Question 135.
Why is sulphuric acid a strong acid?
Answer:
Sulphuric acid ionises in an aqueous solution in two steps as follows :

The larger value of Ka (Ka >10) shows more dissociation of the acid into H3O+ and H2SO4. Thus H2SO4 is a strong acid.
Question 136.
What is the action of hot and concentrated sulphuric acid on the following :
(i) Carbon
(ii) Sulphur
(iii) Phosphorus
(iv) Zn
(v) Cu
(vi) Haloacid (HX)?
Answer:

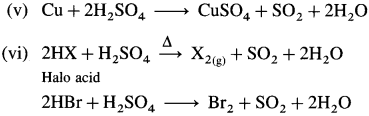
![]()
Question 137.
Write the reactions of the following with concentrated H2SO4 :
(i) NaCl
(ii) KNO3
(iii) CaF2.
Answer:
(i) NaCl + H2SO4conc. → NaHSO4 + HCl
(ii) 2KNO3 + H2SO4conc. → K2SO4 + 2HNO3
(iii) CaF2 + H2SO4conc. → CaSO4 + 2HF.
Question 138.
What is the action of hot and concentrated H2SO4 on
(i) FeSO4 and
(ii) HI?
Answer:
- FeSO4 is oxidised to Fe2(SO4)3 by hot and concentrated H2SO4.
\(2 \mathrm{FeSO}_{4}+2 \mathrm{H}_{2} \mathrm{SO}_{4} \stackrel{\Delta}{\longrightarrow} \mathrm{Fe}_{2}\left(\mathrm{SO}_{4}\right)_{3}+\mathrm{SO}_{2}+2 \mathrm{H}_{2} \mathrm{O}\) - HI is oxidised to I2 by hot and concentrated H2S04.
2HI + H2SO4 → 2H2O + SO2 + I2
Question 139.
Complete the following reactions :
(i) Fe + dil. H2SO4
(ii) Benzene + H2SO2(conc.)
(iii) PCl5 + H2SO4
(iv) K4[Fe(CN)6]
(v) KClO3
(vi) Fluorspar, CaF2 + dil. H2SO4.
Answer:

Question 140.
In the dissociation of H2SO4 in water, why is the second dissociation constant smaller than the first?
Answer:
- H2SO4 is a dibasic acid. In aqueous, solution it dissociates in two steps as follows :
H2SO4(aq) ⇌ H+(aq) + HSO–4(aq) Ka1 > 10
HSO; (aq) ⇌ H+(aq) + SO2-4(aq) Ka2 = 1.2 x 10-2
- Neutral H2SO4 molecule has more tendency to lose proton (H+) than anionic Lowry-Bronsted base \(\mathrm{HSO}_{4}^{-} \text {. }\)
- Therefore second dissociation constant Ka2 is smaller than first dissociation constant Ka1.
![]()
Question 141.
Mention the conditions to maximize the yield of H2SO4 by contact process.
Answer:
- In the contact process of the manufacture of sulphuric acid, SO2 is oxidised to SO3 by heating the mixture on the heterogeneous catalyst V2O5.
2SO2(g) + O2(g) ⇌ 2SO3(g) ΔH= – 196.6 kJ - The forward reaction is exothermic and there is a decrease in number of moles and volume.
- The optimum conditions to maximise the yield of H2SO4 are pressure of 2 bar and temperature around 720 K.
Question 142.
What are the uses of H2SO4?
Answer:
H2SO4 has following uses :
- In the preparation of HNO3, HCl, H2PO4, Na2CO3 sulphates, alums, alcohols, ethers, etc.
- In the manufacture of dyes, fertilizers like ammonium sulphate, super phosphate, detergents.
- As an electrolyte in lead storage battery.
- As a dehydrating agent.
- As an oxidising agent.
- For refining petroleum.
- As a pickling agent for removing layers of basic oxides from the metal surfaces like Fe, Cu, etc. before the metals are galvanized, electroplated, etc.
- It is used as a laboratory reagent.
- It is used in the manufacture of nitrocellulose products.
Question 143.
What happens when,
(i) SO3 gas is passed through water
(ii) Concentrated sulphuric acid is added to sugar?
Answer:
(i) SO3 dissolves in water and forms sulphuric acid, H2SO4.
SO3(g) + H2O(1) → H2SO4(aq)
(ii) Concentrated sulphuric acid when added to sugar, it is dehydrated giving carbon.
![]()
Question 144.
How is chlorine obtained from HCl?
Answer:
Chlorine is obtained by the oxidation of hydrochloric acid by various oxidising agents:
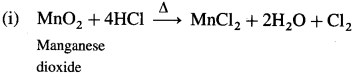

Question 155.
How is chlorine obtained from rock salt or NaCl?
Answer:
Rock salt or NaCl in the presence of MnO2 and concentrated H2SO4 forms chlorine.
The reaction takes place in two steps.

Question 156.
How is chlorine manufactured by Deacon’s process?
Answer:
In Deacon’s process, chlorine is manufactured by oxidising hydrogen chloride gas by atmospheric oxygen in the presence of CuCl2 as a catalyst at 723 K.
![]()
![]()
Question 147.
How is chlorine manufactured by the electrolytic process?
Answer:
- On a large scale chlorine is obtained by an electrolytic process.
- The electrolyte is brine, a concentrated solution of NaCl.
- Nelson’s two compartments diaphragm cell is used for electrolysis in which stout graphite rod is used as an anode while U shaped steel vessel is used as a cathode.
- Reactions :
NaCl → Na+ + Cl–
H2O ⇌ H++ OHAt cathode :
2H2O + 2e– → H2 + 20H–
Na+ +OH– → NaOHAt anode :
2Cl → 2Cl + 2e–
2Cl → Cl2(g)
Question 148.
What are the physical properties of chlorine?
Answer:
The physical properties of chlorine are as follows :
- It is greenish-yellow gas with a suffocating and pungent smell and is heavier than air.
- It is a poisonous gas.
- When liquefied, chlorine forms a greenish-yellow liquid which has boiling point 239 K.
- Chlorine is soluble in water and the solution is called chlorine water.
Question 149.
What is the action of chlorine on the following :
(i) Na
(ii) K
(iii) Ca
(iv) Fe
(v) A1
(vi) Cu?
Answer:
(i) 2Na + Cl2 → 2NaCl
(ii) 2K + Cl2 → 2KCl
(iii) Ca + Cl2 → CaCl2
(iv) 2Fe + 3Cl2 \(\stackrel{\Delta}{\longrightarrow}\) 2FeCl3
(v) 2A1 + 3Cl2 \(\stackrel{\Delta}{\longrightarrow}\) 2A1Cl3
(vi) Cu + Cl2 → CuCl2
Question 150.
What is the action of Cl2 on the following :
(i) P4
(ii) As
(iii) Sb
(iv) B
(v) S.
Answer:
(i) P4 + 6Cl2 → 4PCl3 and P4 + 10Cl2 → 4PCl5
(ii) 2As + 3Cl2 → 2AsCl3
(iii) 2Sb + 3Cl2 → 2SbCl3
(iv) 2B + 3Cl2 → 2BCl3
(v) S8 + 4Cl2 → 4S2Cl2
Sulphur monochloride
![]()
Question 151.
What is the action of chlorine on
(i) hydrogen
(ii) hydrogen sulphide?
OR
Give two reactions to show the affinity of hydrogen towards chlorine.
Answer:
(i) Since chlorine has high affinity for hydrogen, it forms hydrogen chloride in the presence of sunlight.
\(\mathrm{H}_{2}+\mathrm{Cl}_{2} \stackrel{h v}{\longrightarrow} 2 \mathrm{HCl}\)
(ii) Chlorine reacts with hydrogen sulphide to form hydrogen chloride and sulphur.
H2S + Cl2 → 2HCl + S
Question 152.
What is the reaction of chlorine with ammonia?
Answer:
- Chlorine reacts with the excess of ammonia to form ammonium chloride, NH4Cl and nitrogen.
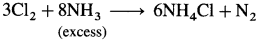
- When chlorine is in excess, then with ammonia it forms explosive nitrogen trichloride, NCl3.
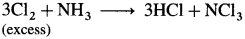
Question 153.
Explain the reactions of chlorine with alkalies.
Answer:
- Chlorine with cold and dilute caustic soda, NaOH forms sodium chloride and sodium hypochlorite, NaOCl.
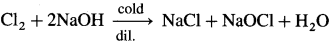
- Chlorine with hot and concentrated NaOH forms sodium chloride and sodium chlorate, NaClO3.

- When chlorine is passed over dry slaked lime Ca(OH)2, bleaching powder, CaOCl2 is obtained.
Ca(OH)2 + Cl2 → CaOCl2 +H2O
Question 154.
What is the action of chlorine on
(i) methane and
(ii) ethylene?
Answer:
(i) With methane, chlorine forms substituted products.
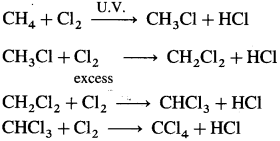
(ii) With unsaturated hydrocarbons, chlorine forms addition products.

Question 155.
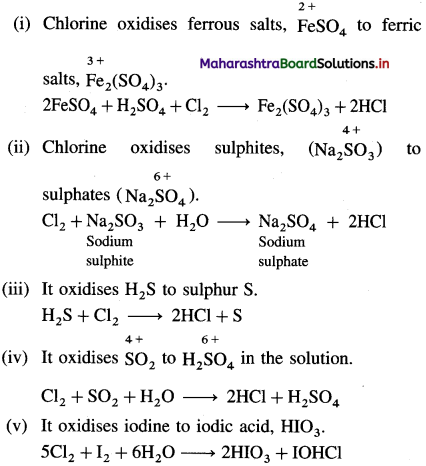
Explain oxidising nature of chlorine with chemical reactions.
Answer:
![]()
Question 156.
Explain the bleaching action of chlorine.
Answer:
(i) Chlorine acts as a powerful bleaching agent due to its oxidising nature.
(ii) In moist conditions or in the presence of water it forms unstable hypochlorous acid, HOCl which decomposes giving nascent oxygen which oxidises the vegetable colouring matter of green leaves, flowers, litmus, indigo, etc.
Cl2 + H2O → HCl + HOCl
HOCl → HCl + [O]
Vegetable coloured matter + [O] → colourless matter.
Question 157.
Explain disinfecting action of chlorine.
OR
Chlorine is used for the sterilization of water. Explain.
Answer:
(i) Since chlorine has an ability of killing harmful micro-organisms, it acts as a good disinfecting agent.
(ii) This ability is due to the oxidising nature of chlorine which produces nascent oxygen in aqueous solutions.
H2O + Cl2 → HCl + HOCl
HOCl → HCl + [O]
Question 158.
What is the action of Cl2 on
(a) Cold and dil. NaOH
(b) Hot and cone. NaOH
(c) P4 molecule
(d) Iron (II)
Answer:

Question 159.
Write the formulae of tear gas, phosgene and mustard gas.
Answer:

![]()
Question 160.
Who prepared hydrochloric acid first?
Answer:
Glauber in 1648, first prepared hydrochloric acid by heating common salt NaCl with concentrated H2SO4.
\(2 \mathrm{NaCl}+\mathrm{H}_{2} \mathrm{SO}_{4}(\text { conc. }) \stackrel{\Delta}{\longrightarrow} \mathrm{Na}_{2} \mathrm{SO}_{4}+2 \mathrm{HCl}\)
Question 161.
What are the physical properties of hydrogen chloride gas?
Answer:
- Hydrogen chloride is a colourless gas with a pungent odour.
- It is heavier than air.
- Hydrogen chloride on liquefication forms a colourless liquid having boiling point 189 K and on freezing it forms white solid crystals having melting point 159 K.
- Hydrogen chloride is highly soluble in water.
Question 162.
What is hydrochloric acid?
Answer:
An aqueous solution of hydrogen chloride is called hydrochloric acid.
It is acidic in nature. It is a strong acid and dissociates almost completely.
HCl(aq) + H2O → H3O+(aq) + Cl–(aq) Ka = 107
Question 163.
What is aquaria? What is the action of aquaria on (i) Au and (ii) Pt?
Answer:
A mixture of three parts of concentrated HCl solution and one part of concentrated HNO3 solution is known as aquaria.
Aquaregia dissolves almost all substances including noble metals like gold, platinum.
This high solubility is due to the formation of nascent chlorine.

Question 164.
Write the reactions of hydrochloric acid with the following :
(a) NaHSO3
(b) NaHCO3
(c) CaO
(d) Mg(OH)2
(e) NaOH.
Answer:
(a) NaHSO3 + HCl → NaCl + H2O + SO2
(b) NaHCO3 + HCl → NaCl + H2O + CO2
(c) CaO + 2HCl → CaCl2 + H2O
(d) Mg(OH)2 + 2HCl → MgCl2 + 2H2O
(e) NaOH + HCl → NaCl + H2O
Question 165.
What are the uses of hydrogen chloride (hydrochloric acid)?
Answer:
Hydrogen chloride (OR hydrochloric acid) is used :
- in the manufacture of chlorine and ammonium chloride,
- to manufacture glucose from com, starch
- to manufacture dye
- in mediClne and galvanising
- as an important reagent in the laboratory
- to extract glue from bones and for the purification of bone black.
- for dissolving metals, Fe + 2HCl(aq) → FeCl2 + H2(g)
![]()
Question 166.
What are the general compositions of interhalogen compounds?
Answer:
(i) The general compositions of interhalogen compounds are XX’, XX’3, XX’5 and XX’7 where halogen X is more electropositive and has larger size than another halogen X’. For example,
| XX’ | XX’3 | XX’5 | XX’7 |
| CIF BrF BrCl ICl | C1F3 BrF3 If3 IC13 (unstable) | C1F5 BrF5 If5 | IF7 |
(ii) As the ratio of radii of X and X’ increases, the number of atoms of X’ per molecule of interhalogen compound increases. For example, iodine having the largest size with fluorine of the smallest size forms stable IF7.
Question 167.
Which are the different types of interhalogen compounds?
Answer:
(i) The general compositions of interhalogen compounds are XX’, XX’3, XX’5 and XX’7 where halogen X is more electropositive and has larger size than another halogen X’. For example,
| XX’ | XX’3 | XX’5 | XX’7 |
| CIF BrF BrCl ICl | C1F3 BrF3 If3 IC13 (unstable) | C1F5 BrF5 If5 | IF7 |
(ii) As the ratio of radii of X and X’ increases, the number of atoms of X’ per molecule of interhalogen compound increases. For example, iodine having the largest size with fluorine of the smallest size forms stable IF7.
Question 168.
What are general characteristics of interhalogen compounds?
Answer:
The general characteristics of interhalogen compounds are as follows :
- In XX’n, X is the halogen which has larger size and is more electropositive, while X’ is the halogen having smaller size and is less electropositive, n is the number of atoms of X’ attached to X.
- Interhalogen compounds are named as halogen halides. The more electropositive halogen is named as such and the less electropositive halogen is named as the halide. In ClF, since Cl is larger and more electropositive than F, the interhalogen compound is named as Chlorine monofluoride,
- As the ratio of radii (radius of X : radius of X’ ) between the atoms X and X’ increases, the number of halogen atoms (n) per interhalogen compound also increases.
- The interhalogen compounds have even number of atoms i.e. 2,4,6,8. For example, ClF3 has 4 atoms and BrF5 has 6 atoms.
- The number of X’ atoms in the interhalogen compounds are always odd.
- The properties of interhalogen compounds are generally intermediate between those of the halogens from which they are made.
- The oxidation state of the atom X in XX’n, is equal to +1, +3, +5, +7 and that of X’ is -1.
- Since the electronegativity difference between two different halogens is low, the interhalogen compounds are covalent in nature.
- Interhalogen compounds exist as gases, liquids and solids depending upon their composition. They are volatile and less stable but not explosive.
- They are diamagnetic in nature.
- Since the interhalogen bond (X – X’) is weaker than parent halogens, they are more reactive than halogens.
x- l so >oui bruin Timor! N
Question 169.
How are interhalogen compounds prepared?
Answer:
All interhalogen compounds are prepared by direct reaction of halogens or reaction of halogen on lower interhalogen compounds. The compound formed depends upon specific conditions of the reactions,
![]()
(a) By direct combination :
- Chlorine monofluoride, ClF : It is a colourless gas obtained by reacting equal volumes of Cl2 and F2.
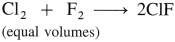
- Chlorine trifluoride, ClF3 : It is a colourless gas, obtained by reacting Cl2 with excess of F2.
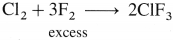
- Bromine trifluoride, BrF3 : It is a yellow green liquid obtained by reacting Br2.
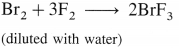
- Iodine trichloride, ICl3 : It is an orange solid obtained by reacting I2 with excess of Cl. This orange solid dimerises to form I2Cl6 having Cl-bridges.
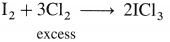
- Iodine chloride, ICl : It is obtained by reaction between equimolar amounts of I2 and Cl2. a- form of it is a ruby red solid while 1-form is a brown-red solid.

- Bromine pentafluoride, BrF5 : It is a colourless liquid and obtained by the reaction of bromine with the excess of fluorine.
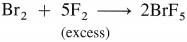
- Bromine chloride BrCl : It is a gas, obtained by the reaction of bromine with chlorine taken in equal volumes.

(b) By the reaction of halogen with interhalogen compound :
- Bromine fluoride (BrF) : It is a pale brown coloured gas prepared by the action of Br2 on BrF3. Br2 + BrF3 → 3BrF
- Bromine trifluoride ( BrF3) : BrF3 can also be prepared by the action of Br2 on ClF’3.
Br2 + ClF3 → 2BrF3 + BrCl
(c) SpeClal reaction for the preparation of ICl :
- ICl( Wij’s solution) can be prepared by the action of Iodinef (I2) on potassium chlorate (KClO3)
I2 + KClO3 → ICl + KlO3
Question 170.
Give the physical states of the following molecules :
BrCl, IBr, IF5, BrF5, ClF3, IF7
Answer:
| Molecules | Physical states |
| BrCl | Gas |
| IBr | Black solid |
| If5 | Colourless gas |
| BrF5 | Colourless liquid |
| ClF3 | Colourless gas |
| IF7 | Colourless gas |
Question 171.
Discuss the hydrolysis reactions given by interhalogen compounds.
Answer:
Interhalogen compounds undergo hydrolysis and form haloacids of lower halogen and oxyacid of higher halogen in interhalogen compounds.
BrCl + H2O → HOBr + HCl
5ICl + 3H2O → HIO3 + 5HCl + 2I2
2ICI3 + 3H2O → HIO3 + 5HCl + ICl
Question 172.
Give the addition reaction given by interhalogen compounds.
Answer:
Interhalogen compounds give addition reactions with unsaturated hydrocarbons.
CH2 = CH2 + XX’ → CH2X – CH2X’
CH2 = CH2 + ICl → CH2I – CH2Cl
![]()
Question 173.
Arrange the given compounds in the order of their thermal stability. ClF, IBr, BrCl, ICl.
Answer:
The thermal stability of interhalogen compounds depends upon the electronegativity of the combining atoms.
| Elements | F | Cl | Br | I |
| Electronegativity | 4.0 | 3.2 | 3.0 | 2.7 |
More the difference between the electronegativity the combining atoms, more is the thermal stability. Hence the order of thermal stability is ClF > ICl > IBr > BrCl
Question 174.
Give one fluorinating reaction given by inter-halogen compounds.
Answer:
Interhalogen compounds like ClF3, BrF3, etc. are very reactive and they are strong fluorinating agents.
U(S) + 3ClF3(1) → UF6(g) + 3ClF(g)
Question 175.
What are the uses of interhalogen compounds?
Answer:
The uses of interhalogen compounds are as follows :
- ICl is used as a halogenating agent and also in the estimation of iodine number of fats and oils.
- In the preparation of polyhalides, interhalogen compounds are used.
- ClF3 and BrF3 are widely used as fluorinating agent. They are used in the separation of 235U isotope by fluorinating.
235U(S) + 3ClF3(l) – 235UF6(g) + 3ClF(g) - In propellants ClF3 and IF3 are used as oxidisers.
- They are used as non-aqueous solvents.
- The XX’ interhalogen compounds are used as a catalyst for the oxidation of As(III)
Question 176.
Give the disproportionation reactions given by the following :
(i) BrF
(ii) ClF3
(iii) ICl3
Answer:

Question 177.
Give hybridisation and geometry of the following molecules :
(1) C1F3
(2) IF5
(3) IF7.
Answer:
| Molecule | Hybridisation | Geometry |
| (1) C1F3 | sp3d | Trigonal bipyramidal or T-shaped |
| (2) IF5 | sp3d2 | Square pyramidal |
| (3) IF7 | sp3d3 | Pentagonal bipyramidal |
Question 178.
Draw structure of :
(a) Chlorine trifluoride
(b) Chlorine pentafluoride
(c) Bromine trifluoride
(d) Iodine dichloride
Answer:
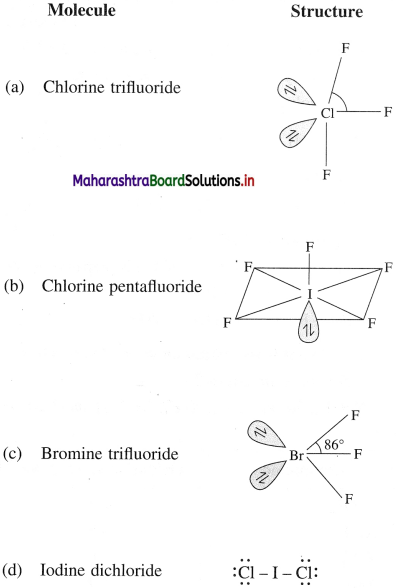
![]()
Question 179.
Complete the given table :
| The oxidation state of the central atom | Number of lone pairs of electrons | Examples |
| + 7 | ……………………………….. | IF7 |
| ……………………………….. | 1 | IF5 |
| ……………………………….. | 2 | I2Cl6 |
| + 1 | ……………………………….. | IBr |
Answer:
| The oxidation state of the central atom | Number of lone pairs of electrons | Examples |
| + 7 | 0 | IF7 |
| + 5 | 1 | IF5 |
| + 3 | 2 | I2Cl6 |
| + 1 | 3 | IBr |
Question 180.
Explain IC1 is more reactive than I2.
Answer:
- in interhalogen compound ICl and I2 the atoms are bonded by covalent bonds.
- The covalent bond between dissimilar atoms, I and Cl atoms in ICl is weaker than between similar atoms in I2.
- Therefore bond dissociation enthalpy of ICl bond is less than that of I2 bond.
Hence ICl is more reactive than I2.
Question 181.
What is the action of
(i) H2O and
(ii) PF5 on XeF2?
Answer:
(i) Action of H2O ( Hydrolysis) :
XeF2 undergoes hydrolysis to form HF.
2XeF2 + 2H2O → 4HF + 2Xe + O2
(ii) Reaction with PF5 :
XeF2 forms adducts on reaction with PF5.
XeF2 + PF5 → XeF2.PF5
Question 182.
Give the method of preparation of XeF4 and its structure.
Answer:
For the preparation of XeF4 :
The covalent bond between dissimilar atoms, I and Cl atoms in ICl is weaker than between similar atoms in I2.
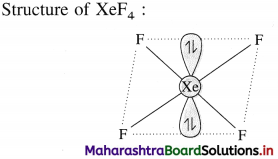
Question 183.
Complete the following :
(1) XeF4 + H2O → ……………… + HF
Answer:
XeF4 + H2O → XeOF2 + 2HF
![]()
Question 184.
What are the uses of :
(1) Helium
(2) Neon
(3) Argon.
Answer:
(1) Uses of helium (He) :
- A mixture of helium (85%) and oxygen (15%) is used for filling balloons.
- A mixture of helium and oxygen is also used for respiration by sea divers instead of air because helium is less soluble in blood than nitrogen under high pressure. It is also used for treatment of asthma.
- Helium is used in produClng inert atmosphere in metallurgical operations and welding of metals.
- Liquid helium is used in produClng low temperature required for research.
- Helium is also used in produClng lasers in low temperature gas thermometry.
- It is used in magnetic resonance imaging.
- It is used as a shielding gas for arc welding, (viii) It is used in supersonic wind tunnels.
- Helium nucleus is used as a bombarding particle for disintegration of atoms.
(2) Uses of neon (Ne) :
- Neon is used in the production of neon discharge lamps and signs by filling Ne in glass discharge tubes.
- Neon signs are visible from a long distance and also have high penetrating power in mist or fog.
- A mixture of neon and helium is used in voltage stabilizers and current rectifiers.
- Neon is also used in the production of lasers and fluorescent tubes.
(3) Uses of argon (Ar) :
- Argon is used to fill fluorescent tubes and radio valves.
- It is used to provide inert atmosphere for welding and production of steel.
- It is used along with neon in neon sign lamps to obtain different colours.
- A mixture of 85% Ar and 15% N2 is used in electric bulbs to enhance the life of the filament.
Question 185.
Helium and neon do not form compounds with fluorine. Why?
Answer:
Helium and Neon do not contain d-orbitals in their respective valence shells and hence their electrons cannot be promoted to higher energy levels like other elements in group 18. Therefore, helium and neon do not form compounds with fluorine.
Multiple Choice Questions
Question 223.
Select and write the most appropriate answer from the given alternatives for each sub¬question :
1. Oxygen molecule shows :
(a) Paramagnetism
(b) Diamagnetism
(c) Ferromagnetism
(d) ferrimagnetism
Answer:
(a) paramagnetism
2. Among group 16 elements, the radioactive element is
(a) Ra
(b) Rn
(c) Bi
(d) Po
Answer:
(d) Po
3. Hybridization involved in H2O is
(a) sp
(b) sp3
(c) sp3d2
(d) sp2
Answer:
(b) sp3
4. The structure of SF4 is
(a) Octahedral
(b) Bipyramidal
(c) Square planar
(d) Tetrahedral
Answer:
(b) Bipyramidal
5. Which one has the highest bond energy?
(a) 0-0
(b) S-S
(c) Se-Se
(d) Te-Te
Answer:
(b) S – S
![]()
6. Which of the following compounds contains S = O as well as S = S bonds?
(a) Sulphuric acid
(b) Thiosulphuric acid
(c) Sulphurous acid
(d) Thiosulphurous acid
Answer:
(b) Thiosulphuric acid
7. Ozone is :
(a) A compound of oxygen
(b) An allotropic oxygen
(c) An isotope of oxygen
(d) An isobar of oxygen
Answer:
(b) An allotropic oxyen
8. Which one has lowest boiling point?
(a) H2O
(b) H2S
(C) H2Se
(d) H2Te
Answer:
(b) H2S
9. Maximum covalency of sulphur is :
(a) Four
(b) Six
(c) Three
(d) Two
Answer:
(b) Six
10. Oxygen exhibits-1 oxidation state in :
(a) OF2
(b) H2O2
(c) HClO
(d) H2O
Answer:
(b) H2O2
11. The oxidation state of oxygen in OF2 is
(a) -1
(b) -2
(c) +1
(d) +2
Answer:
(d) +2
12. In the hydrides of group 16 elements, the minimum bond angle is in
(a) H2O
(b) H2S
(C) H2Se
(d) H2Te
Answer:
(d) H2Te
13. In SF6, sulphur atom undergoes hybridisation
(a) sp3
(b) sp3d
(c) sp3d2
(d) sp3d3
Answer:
(c) sp3d2
14. Al2O3 is
(a) acidic oxide
(b) basic oxide
(c) amphoteric oxide
(d) neutral oxide
Answer:
(c) amphoteric oxide
![]()
15. Which of the following is a basic oxide?
(a) SiO2
(b) P4O10
(c) MgO
(d) Al2O3
Answer:
(c) MgO
16. The most stable allotropic form of sulphur is :
(a) Rhombic sulphur
(b) Flowers of sulphur
(c) Platic sulphur
(d) Mono clinic sulphur
Answer:
(a) Rhombic sulphur
17. The atomicity of sulphur in orthorhombic sulphur is –
(a) 8
(b) 6
(c) 4
(d) 2
Answer:
(a) 8
18. The oxidation state of sulphur in peroxy disul- phuric acid is
(a) +2
(b) +3
(c) +4
(d) +6
Answer:
(d) +6
19. In the contact process, the catalyst used is
(a) TiO2
(b) Pt
(c) V2O5
(d) Fe2O3
Answer:
(c) V2O5
20. What is the molecular formula of the oleum?
(a) H2SO3
(b) H2SO4
(c) H2S2O7
(d) H2S2O8
Answer:
(c) H2S2O7
21. The electronic configuration of bromine is
(a) [Ne] 3s23p5
(b) [Kr] 4d105s25ps
(c) [Ar] 3d104s24p5
(d) [Ar]4d105s25p5
Answer:
(c) [Ar] 3d104s24p5
22. The decreasing order for negative values for electron gain enthalpies is
(a) F > Cl > Br
(b) Cl > F > Br
(c) Br > Cl > F
(d) F > Br > Cl
Answer:
(b) Cl > F > Br
23. The molecular formula H2S2O2 represents which oxoacid among the following?
(a) Hydrosulphurous acid
(b) Thiosulphurous acid
(c) Sulphuric acid
(d) Pyrosulphurous acid
Answer:
(b) Thiosulphurous acid
![]()
24. General electronic configuration of group 16 elements is
(a) ns2np3
(b) ns2np4
(c) ns2np2
(d) ns2np5
Answer:
(b) ns2np4
25. The decreasing order of electronegativity is
(a) Cl > I > Br
(b) Br > I > F
(c) F > Br > I
(d) Cl > F > Br
Answer:
(c) F > Br > I
26. The increasing order of acidic strength of haloacids is
(a) HF > HCl > HBr > HI
(b) HCl > HF > HBr > HI
(c) HCl < HF < HBr < HI
(d) HF < HCl < HBr < HI
Answer:
(d) HF < HCl < HBr < HI
27. Amongst haloacids, the strongest reducing agent is
(a) HCl
(b) HF
(c) HI
(d) HBr
nswer:
(c) HI
28. Haloacid with the maximum thermal stability is
(a) HI
(b) HCl
(c) HF
(d) HBr
Answer:
(c) HF
29. The bleaching action of chlorine is due to
(a) oxidation
(b) reduction
(c) neutralisation
(d) double decomposition
Answer:
(a) oxidation
30. The halogen which does not form polyhalide ion is
(a) F
(b) Br
(c) I
(d) Cl
Answer:
(a) F
31. Camalite is a natural source of
(a) F
(b) Cl
(c) Br
(d) I
Answer:
(b) Cl
32. In Deacon’s process the catalyst used is
(a) V2O5
(b) CuCl2
(c) PtCl4
(d) FeCl3
Answer:
(b) CuCl2
![]()
33. Chlorine on reaction with carbon disulphide forms
(a) CCl4
(b) SO2Cl2
(c) S2Cl2
(d) CSCl2
Answer:
(c) S2Cl2
34. When chlorine is passed over dry slaked lime the product obtained is
(a) CaCl2
(b) HCl
(c) CaOCl2
(d) HOCl
Answer:
(c) CaOCl2
35. Hybridisation in ClF3 is
(a) sp3
(b) sp3d
(c) dsp3
(d) sp3d2
Answer:
(b) sp3d
36. The geometry of ClF3 is
(a) tetrahedral
(b) trigonal bipyramidal
(c) square planar
(d) triangular
Answer:
(b) trigonal bipyramidal
37. The structure of interhalogen compound XX’5 is
(a) square pyramidal
(b) trigonal bipyramidal
(c) tetrahedral
(d) pentagonal bipyramidal
Answer:
(a) square pyramidal
38. Interhalogen compound used as a fluorinating agent is
(a) IF7
(b) BrF5
(c) IF5
(d) ClF3
Answer:
(d) ClF3
39. The increasing order of thermal stability of oxoacids of halogens is
(a) HClO2 < HCIO3 < HClO4
(b) HClO4 < HCIO3 < HClO2
(c) HClO4 < HClO2 < HCIO3
(d) HCIO3 < HClO2 < HClO4
Answer:
(a) HClO2 < HCIO3 < HClO4
40. The shape of IF7 molecules is :
(a) Tetrahedral
(b) Octahedral
(c) Trigonal bipyramidal
(d) Pentagonal bipyramidal
Answer:
(d) Pentagonal bipyramidal
41. The strongest oxidizing agent is :
(a) HOCl
(b) HClO4
(c) HClO3
(d) HClO2
Answer:
(b) HClO4
![]()
42. Iodine can exist in the oxidation states
(a) +1, +3, +5
(b) -1, +1, +3
(c) +3, +5, +7
(d) -1, +1, +3, +5, +7
Answer:
(d) -1, +1, +3, +5, +7
43. Fluorine does not show positive oxidation state due to absence of:
(a) d-orbitals
(b) s-orbitals
(c) p-orbitals
(d) f-orbitals
Answer:
(a) d-orbitals
44. The most powerful oxidising agent is :
(a) Fluorine
(b) Chlorine
(c) Iodine
(d) Bromine
Answer:
(a) Fluorine
45. Which one is the strongest reducing agent?
(a) HF
(b) HCl
(c) HBr
(d) HI
Answer:
(d) HI
46. Fluorine can exist in the oxidation state :
(a) – 1 only
(b) – 1 and + 1
(c) 1-, +1, +3 only
(d) -1, +l,. + 3, +7
Answer:
(a) – 1 only
47. Which of the following halogen is radioactive in nature?
(a) Chlorine
(b) Bromine
(c) Iodine
(d) Astatine
Answer:
(d) Astatine
48. The electronic configuration of krypton is
(a) [Ar] 3d10 As2 Ap6
(b) [Ne] 3s2 3p6
(c) [Ai] 4d10 5s2 5p6
(d) [Xe] 6s2 6p6
Answer:
(a) [Ar] 3d10 As2 Ap6
49. Which one of the following does not exist?
(a) HeF4
(b) XeF4
(c) CF4
(d) SF6
Answer:
(a) HeF4
50. Name the noble gas not present in the air.
(a) Radon
(b) Argon
(c) Krypton
(d) Helium
Answer:
(a) Radon
![]()
51. Which is the most abundant noble gas?
(a) Argon
(b) Helium
(c) Neon
(d) Krypton
Answer:
(a) Argon
52. A mixture used for respiration by the sea divers is OR Which mixture is used for respiration by deep sea divers?
(a) He + O2
(b) Ne + O2
(c) Ar + O2
(d) Kr + O2
Answer:
(a) He + O2
53. Iodine exists as-
(a) polar molecular solid
(b) ionic solid
(c) nonpolar molecular solid
(d) hydrogen-bonded molecular solid
Answer:
(c) nonpolar molecular solid
54. Hybridisation in H2S is
(a) sp
(b) sp3
(c) sp3d2
(d) sp2
Answer:
(b) sp3
55. Noble gas which finds the use as a cryogenic agent is
(a) He
(b) Ne
(c) Ar
(d) Kr
Answer:
(a) He
56 Which of the following compounds of chlorine is used as refrigerant?
(a) CCl3NO2
(b) CCl2F2
(c) COCl2
(d) CCl4
Answer:
(b) CCl2F2
![]()
57. The increasing order of oxidising power of oxyacids of chlorine is
(a) HClO2 > HClO3 > HClO4
(b) HOCl > HCl02 > HClO4
(c) HClO2 < HClO3 < HClO4
(d) HClO4 < HOCl < HCO2
Answer:
(c) HClO2 < HClO3 < HClO4
58. S-O-O-S bonds are present in
(a) H2S2O6
(b) H2SO5
(c) H2S2O5
(d) H2S2O8
Answer:
(d) H2S2O8
59. The increasing order of thermal stability of hydrides of sixteenth group elements is
(a) H2O < H2S < H2Se < H2Te
(b) H2S < H2O < H2Se < H2Te
(c) H2Te < H2S < H2Se < H2O
(d) H2Te < H2Se < H2S < H2O
Answer:
(d) H2Te < H2Se < H2S < H2O
60. The bond angle F-Cl-F in ClF3 is
(a) 89°
(b) 109°29
(c) 95°
(d) 120°
Answer:
(a) 89°
61. The oxidation number of Xe in XeOF2 is :
(a) O
(b) +2
(c) +4
(d) +3
Answer:
(c) +4
62 XeF4 on partial hydrolysis produces
(a) XeF2
(b) XeOF2
(c) XeOF4
(d) XeO3
Answer:
(c) XeOF4
63. Shape of XeO4 is
(a) Octahedral
(b) Square pyramidal
(c) Pyramidal
(d) T-shaped
Answer:
(b) Square pyramidal
![]()
64. In XeF2, XeF4 and XeF6 the number of lone pairs on Xe is respectively
(a) 2, 3, 1
(b) 1, 2, 3
(c) 4, 1, 2
(d) 3, 2, 1
Answer:
(d) 3, 2, 1