Balbharti Maharashtra State Board 12th Chemistry Important Questions Chapter 14 Biomolecules Important Questions and Answers.
Maharashtra State Board 12th Chemistry Important Questions Chapter 14 Biomolecules
Question 1.
What are biomolecules? Give examples of biomolecules.
Answer:
Biomolecules: The lifeless, complex organic molecules which combine in a specific manner to produce life or control biological reactions are called biomolecules.
Examples: Carbohydrates, lipids (fats and oils), nucleic acids, enzymes.
![]()
Question 2.
What is the importance of biomolecules?
Answer:
Biomolecules are organic molecules which combine in a particular fashion to give complex substances which help to sustain life and produce identical daughter cells and play an important role in the actions of an organism.
- Carbohydrates are the major constituents of food and source of energy.
- Proteins help in proper functioning of living beings. They are important constituents of skin, hair, muscles. Enzymes which catalyse chemical reactions that take place in cells are proteins.
- Lipids (fats and oils) function as the storehouses of energy.
- Nucleic acids, the ribonucleic acid (RNA), and deoxyribonucleic acid (DNA) are responsible for genetic characteristics and synthesis of proteins.
Question 3.
What are carbohydrates?
OR
Define the term : Carbohydrates.
Answer:
Carbohydrates : Carbohydrates are optically active polyhydroxy aldehydes or polyhydroxy ketones, or the compounds which on hydrolysis produce polyhydroxy aldehydes or polyhydroxy ketones.
Examples : Glucose, sucrose, fructose.
Question 4.
What is monosaccharide?
Answer:
The basic unit of all carbohydrates which is a simple carbohydrate and cannot be hydrolysed further is known as monosaccharide. The monosaccharide is crystalline and soluble in water. E.g. Glucose, fructose, ribose.
Question 5.
Mention the names of monosaccharides or simple carbohydrates.
Answer:
Monosaccharides are (1) glucose (2) fructose (3) ribose.
Question 6.
State the basic unit of all carbohydrates.
Answer:
The basic unit of all carbohydrates which is a simple carbohydrate and cannot be hydrolysed further is known as monosaccharide.
Question 7.
How are carbohydrates classified?
OR
Classification of carbohydrates with examples.
Answer:
Carbohydrates are classified as monosaccharides oligosaccharides and polysaccharides.
(1) Monosaccharides : These carbohydrates cannot be further hydrolysed into smaller units. They are basic units of all carbohydrates, and are called monosaccharides.
Examples : Glucose, fructose, ribose
(2) Oligosaccharides : An oligosaccharide is a carbohydrate (sugar) which on hydrolysis gives two to ten monosaccharide units.
Depending on the number of monosaccharides produced on hydrolysis, oligosaccharides are further classified as :
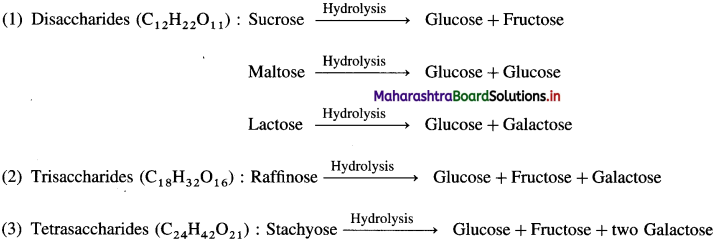
Oligosaccharide is homogeneous. In this, each molecule of oligosaccharide contains the same number of monosaccharide units joined together in the same order as every other molecule of the same oligosaccharide.
(3) Polysaccharides : These are carbohydrates which on hydrolysis give a large number of monosaccharides.
Polysaccharides are tasteless, amorphous, insoluble in water. They are long chain, naturally αcurring polymers of carbohydrates.
Example : Cellulose, starch, glycogen.
![]()
Question 8.
Classify the following carbohydrates into Monosaccharide, Disaccharide, Oligosaccharide, Polysaccharide.
(1) Glucose
(2) Starch
(3) Sucrose
(4) Maltose
(5) Galactose
(6) Lactose
(7) Ribose.
Answer:
| Carbohydrates | Class |
| (1) Glucose | Monosaccharide |
| (2) Starch | Polysaccharide |
| (3) Sucrose | Disaccharide |
| (4) Maltose | Disaccharide |
| (5) Galactose | Monosaccharide |
| (6) Lactose | Disaccharide |
| (7) Ribose | Monosaccharide |
Question 9.
Classify the following carbohydrates.
(1) Cellulose,
(2) Maltose,
(3) Raffinose,
(4) Fructose.
Answer:
| Carbohydrates | Class |
| (1) Cellulose (2) Maltose (3) Raffinose (4) Fructose | Polysaccharide Disaccharide Trisaccharide Monosaccharide |
Question 10.
Classify the following into monosaccharides, oligosaccharides and polysaccharides.
(1) Starch
(2) Glucose
(3) Stachyose
(4) Maltose
(5) Raffinose
(6) Cellulose
(7) Sucrose
(8) Lactose.
Answer:
| Monosaccharides | Glucose |
| Oligosaccharides | Stachyose, maltose, raffinose, sucrose, lactose |
| Polysaccharides | Starch, cellulose |
Question 11.
Classify the following into monosaccharides and disaccharides.
Ribose, maltose, galactose, fructose and lactose (~2 mark each)
Answer:
| Monosaccharides | Ribose, galactose, fructose |
| Disaccharides | Maltose, lactose |
![]()
Question 12.
Give the preparation of glucose from sucrose or cane sugar.
OR
Describe the laboratory method of preparation of glucose.
Answer:
Preparation of glucose from sucrose (cane sugar) : Laboratory method.
Glucose is prepared in the laboratory by hydrolysis of sucrose by boiling it with dilute hydrαhloric acid or dilute sulphuric acid for about two hours. On hydrolysis, sucrose gives one molecule of glucose and one molecule of fructose.
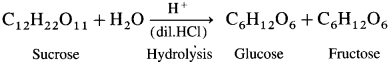
Alcohol is added during cooling to separate glucose and fructose since, glucose is almost insoluble in alcohol, hence it crystallizes out first. Fructose remains in the solution as it is more soluble than glucose.
Crystals of glucose are separated out by filtration and purified by recrystallization.
Question 13.
Give the preparation of glucose from starch.
OR
How is glucose prepared on commercial scale?
Answer:
Commercially, on a large scale, glucose is prepared by hydrolysis of starch with dilute sulphuric acid. Starchy material is mixed with water and dilute sulphuric acid and heated at 393 K under 2 to 3-atm pressure. Starch is hydrolysed to give glucose.

Question 14.
Explain the structure of glucose.
Answer:
Molecular formula of glucose is C6H12O6.
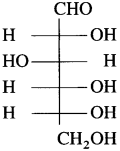
Glucose has an aldohexose structure. In other words, glucose molecule contains one aldehydic, that is, formyl group and the remaining five carbons carry one hydroxyl group (-OH) each. The six carbons in glucose form one straight chain.
Question 15.
Describe the action of following reagents on glucose :
(1) HI
(2) Hydroxyl amine (NH2OH)
(3) Hydrogen cyanide
(4) Bromine water
(5) dil. Nitric acid
(6) Acetic anhydride.
Answer:
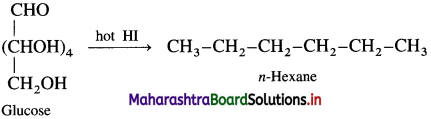
(1) Action of HI : Glucose on prolonged heating with HI gives n-hexane, indicates that all the six carbon atoms are linked in straight chain.
(2) Action of hydroxyl amine : Glucose reacts with hydroxyl amine in an aqueous solution to form glucose oxime. This indicates the presence of CHO group in glucose.
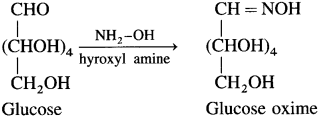
(3) Action of hydrogen cyanide : Glucose reacts with hydrogen cyanide to form glucose cyanohydrin.
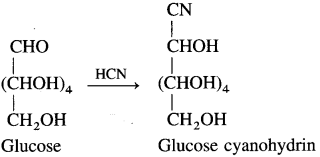
(4) Action of bromine water : Glucose on oxidation with mild oxidising agent like bromine water gives gluconic acid, which shows that the carbonyl group in glucose is aldehyde group.
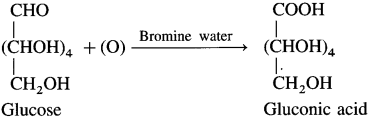
(5) Action of dll. nitric acid : Glucose on oxidation with dilute nitric acid forms dicarboxylic acid, saccharic acid. This indicates the presence of a primary alcoholic group (-CH2OH) in glucose.
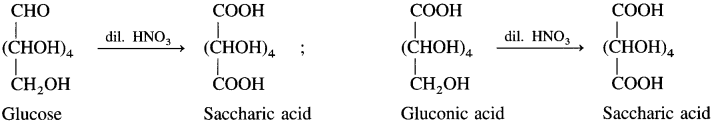
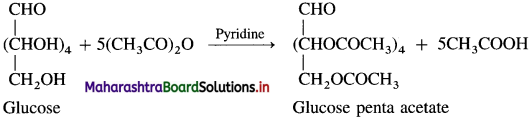
(6) Action of acetic anhydride : When glucose is heated with acetic anhydride in the presence of catalyst pyridine, glucose penta acetate is formed. It indicates that glucose is a stable compound and contains five hydroxyl groups.
![]()
Question 16.
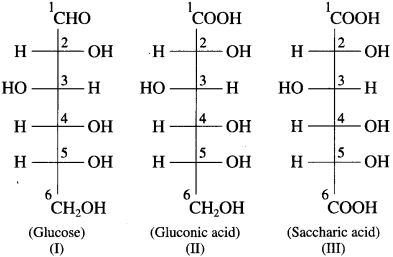
Write Fischer projection formulae for
(1) Glucose
(2) Gluconic acid
(3) Saccharic acid.
Answer:
Fischer projection formulae of glucose, gluconic acid and saccharic acid :
Question 17.
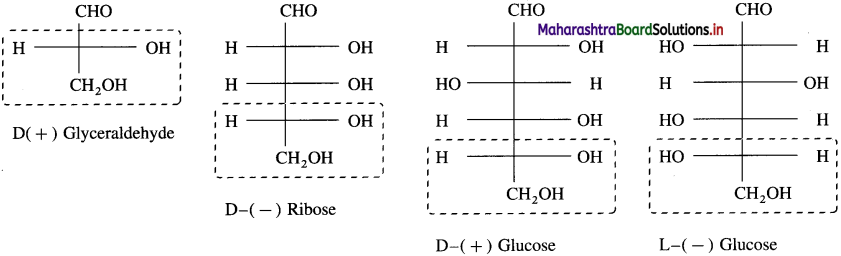
Explain D and L configuration in sugars.
Answer:
The simplest carbohydrates glyceraldehyde is chosen as the standard, to assign D and L configuration to monosaccharides. Glyceraldehyde contains one asymmetric carbon atom and exist in two enantiomeric forms

The dextro entantiomer is represented as (+) glyceraldehyde and it is referred as D-configuration i.e., D-glyceraldehyde. The laevo enantiomer of glyceraldehyde is represented as ( -) glyceraldehyde and it corelated as L-configuration i.e., L-glyceraldehyde.
In Fischer projection formula, a monosaccharide is assigned D-configuration if the (- OH) hydroxyl group at the last chiral carbon and lies towards right hand side. On the other hand it is assigned L-configuration if the – OH group on the last chiral carbon atom and lies on the left hand side. In monosaccharides, the most oxidised carbon (i.e., -CHO) is at the top.
Examples :
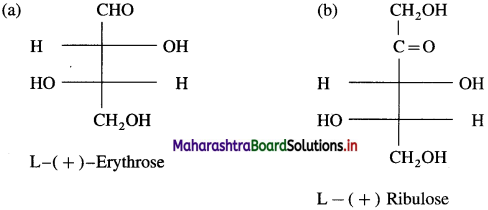
Question 18.
Write Fischer projection formulae for (a) L-( + )-erythrose (b) L-( +) ribulose.
Answer:
![]()
Question 19.
Is the following sugar, D-sugar or L-sugar?

Answer:
The compound is L-sugar.
The compound is L-sugar.
Question 20.
Assign D/L configuration to the following monosaccharides.
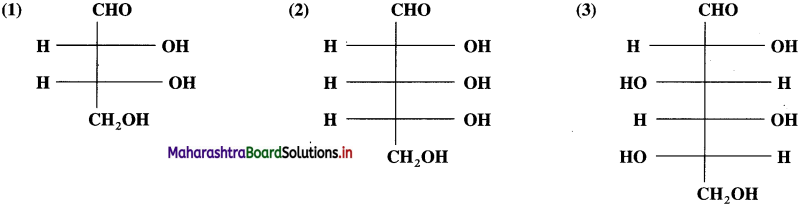
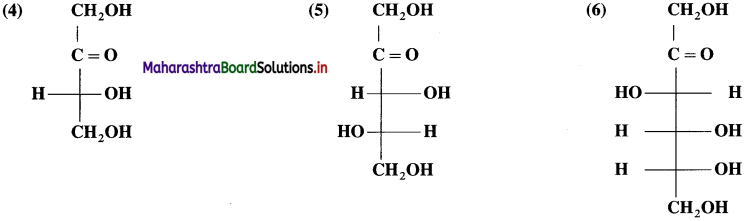
Answer:
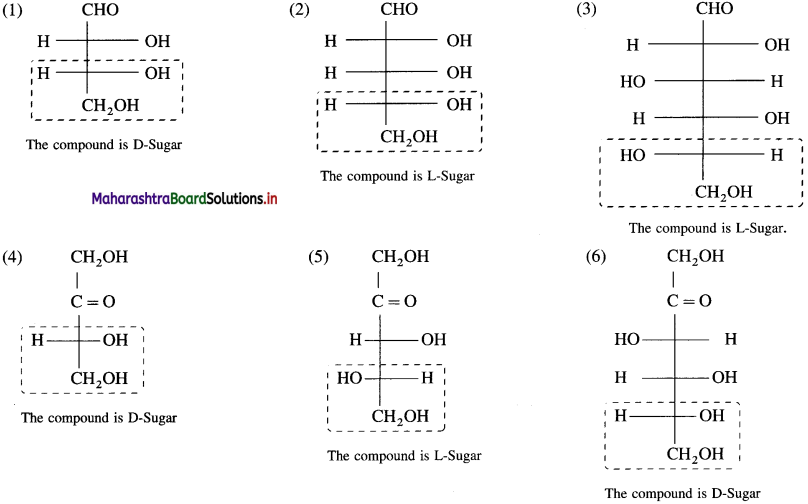
Question 21.
Explain ring structure of glucose.
Answer:
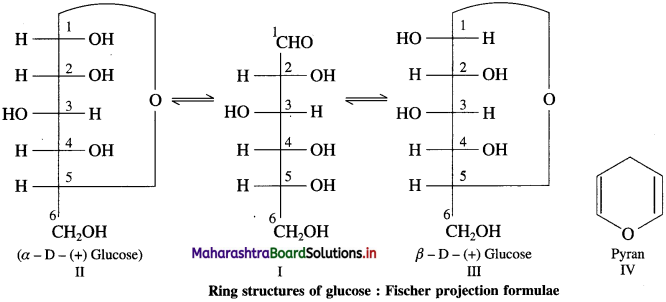
Glucose has two cyclic structures (II and III) which are in equilibrium with each other through the open chain structure (I) in aqueous solution.
![]()
The ring structure of glucose is formed by reaction between the formyl ( – CHO) group and the alcoholic (- OH) group at C – 5. Thus, the ring structure is called a hemiacetal. The two hemiacetal structures (II and III) differ only in the configuration of C – I (Fig.), the additional chiral centre resulting from ring closure. The two ring structures are called α- and β- anomers of glucose and C-l is called the anomeric carbon. The ring of the cyclic structure of glucose contains five carbons and one oxygen. Thus, it is a six membered ring. It is called pyranose structure, in analogy with the six membered heterαyclic compound pyran (IV). Hence glucose is also called glucopyranose.
Question 22.
Write the structures of α-D-( + )-glucopyranose and β-D-( +) glucopyranose.
Answer:
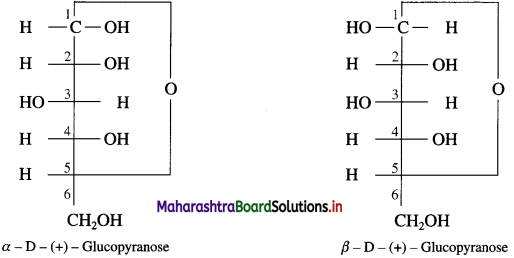
Question 23.
Explain Haworth formula of glycopyranose.
Answer:
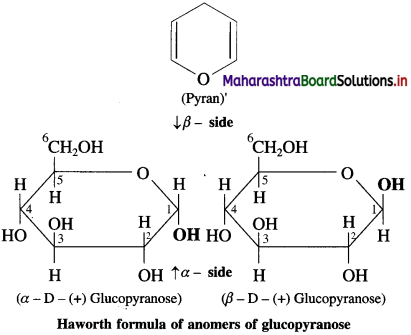
In the Haworth formula the pyranose ring is considered to be in a perpendicular plane with respect to the plane of paper. The carbons and oxygen in the ring are in the places as they appear in figure. The lower side of the ring is called α-side and the upper side is the β-side. The α-anomer has its anomeric hydroxyl (- OH) group (at C-l) on the α-side, whereas the β-anomer has its anomeric hydroxyl (- OH) group (at C-l) on the β-side. The groups which appear on right side in the Fischer projection formula appear on α-side in the Haworth formula, and the groups which appear on left side in the fischer projection formula appear on a β-side in the Haworth formula.
Question 24.
Explain the structure of fructose.
Answer:
Fructose has molecular formula C6H12O6. It contains ketonic functional group at carbon number 2 and six carbon atoms in straight chain. It belongs to D-series and is a laevo rotatory compound. It is written as D-( – )-fructose. Being an α-hydroxy keto compound fructose is a reducing sugar.
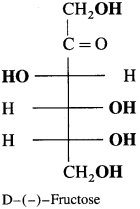
![]()
Question 25.
Draw mirror images of glucose and fructose.
Answer:
(1) Glucose
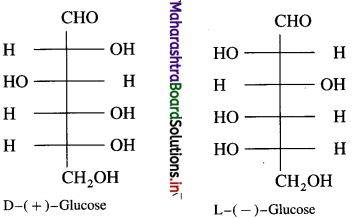
(2) Fructose
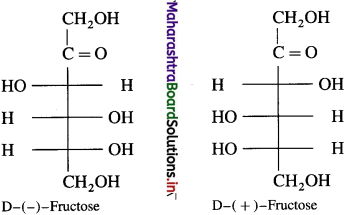
Question 26.
Write the two cyclic structures of α-D-( – )-fructofuranose and β-D-( – )-fructofuranose exist in equilibrium with open chain structure.
Answer:
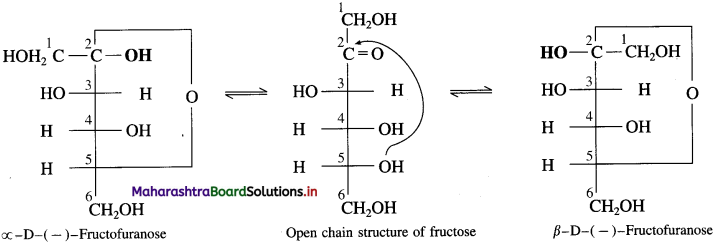
Question 27.
Write the Haworth projection formulae for α -D-( -) – Fructofuranose and β – D – ( -) – Fructo- furanose.
Answer:
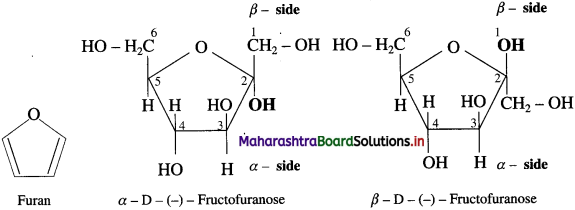
Question 28.
Explain the structure of sucrose.
Answer:
Sucrose is a hexasaccharide and has molecular formula C12H22O11. The structure of shcrose contains glycosidic linkage between C – 1 of α-glucose and C – 2 of β-fructose. Since aldehyde and ketone groups of both monosaccharide units are involved in the formation of glycosidic bond, sucrose is a nonreducing sugar.
Sucrose is dextrorotatory, on hydrolysis with dilute acid or an enzyme invertase gives equimolar mixture of dextrorotatory glucose and laevorotatory fructose.
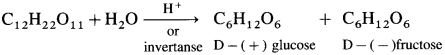
The solution is laevorotatory because laevo rotation of fructose (- 92.4°) is more than dextrorotation of glucose ( + 52.50), hence the sign of rotation is changed from (+) to (-) after hydrolysis, the product is called invert sugar.
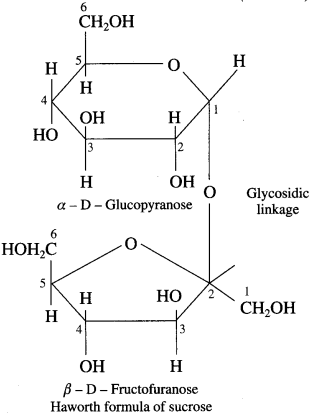
![]()
Question 29.
Explain the structure of maltose.
Answer:
Maltose is another disaccharide obtained by partial hydrolysis of starch or made of two units of D-glucose. In maltose, C-l of one α-D-glucose is linked to C-4 of another α-D-glucose molecule by glycosidic linkage. The glucose ring which uses its hydroxyl group at C-1 is α – 1 → 4 glycosidic linkage. It is a reducing sugar because a free aldehyde group can be produced at C1 of second glucose molecule. Maltose on hydrolysis with dilute acids gives glucose.
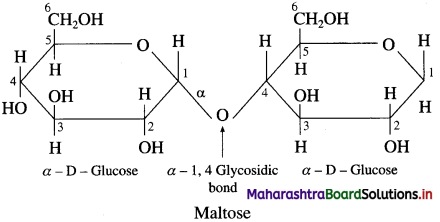
Question 30.
Draw a neat diagram for Haworth formula of maltose.
Question 31.
Explain the structure of lactose.
Answer:
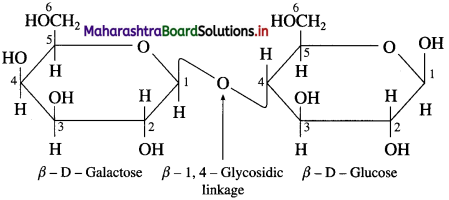
Lactose (C12H22O11) is a disaccharide. It is found in milk, therefore, it is also known as milk sugar. It is formed from two monosaccharide units, namely D – galactose and D – glucose. The glycosidic linkage is formed between C-l of β-D-galactose and C -4 of glucose. Therefore the linkage in lactose is called β – 1,4 – glycosidic linkage. The hemiacetal group at C-l of the glucose unit is not involved in glycosidic linkage but is free. Hence lactose is a reducing sugar. The above figure shows Haworth formula of lactose.
Question 32.
What are the hydrolysis products of (1) lactose (2) sucrose?
Answer:
(1) Lactose on hydrolysis in presence of an acid or enzyme lactase gives one molecule each of glucose and galactose
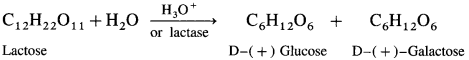
(2) Sucrose on hydrolysis in the presence of dii. acid or the enzyme invertase gives one molecule each of glucose and fructose.

Question 33.
Explain the structure of starch.
Answer:
Starch is found in cereal grains, roots, tubers, potatoes, etc. It is a polymer of α-D-glucose and consists of two components, amylose and amylopectin.
![]()
Amylose is water soluble component forms blue coloured complex with iodine. It constitutes about 20 % of starch. Amylose contains 200 to 1000 α-D-glucose units linked together by glycosidic linkage between C-l of one unit and C-4 of another unit. i.e. α-1, 4 glycosidic linkages.
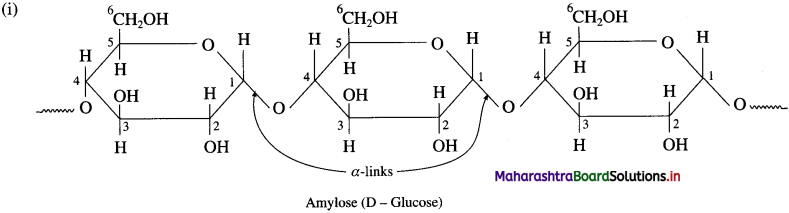
Amylopectin is insoluble in water and constitutes about 80 % starch which forms blue-violet coloured complex with iodine. It is a branched chain polymer. In amylopectin, α-D-glucose molecules are linked together by glycosidic linkage between C1 – of one unit and C-4 of another unit to form long chain and branching αcurs by glycosidic linkage between C-l and C6 glycosidic linkage.
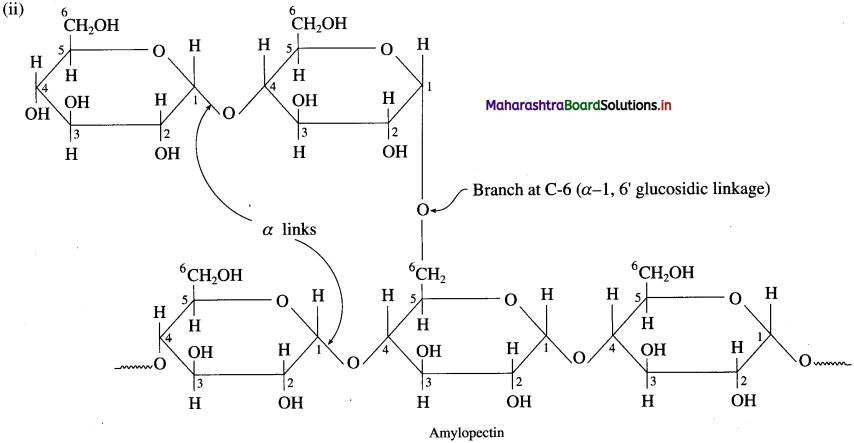
Question 34.
What are polysaccharides?
Answer:
A large number of same or different monosaccharides are joined together by glycosidic linkages are called polysaccharides. They have general formula (C6H10O5)n.
Question 35.
Explain the structure of cellulose.
Answer:
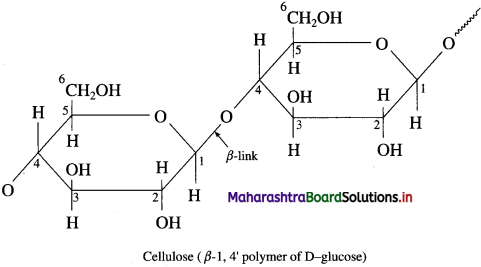
Cellulose mainly αcurs in plants. Cell wall of plant cells is made up of cellulose. It is a long chain polymer. In cellulose, β-D-glucose units are linked by glycosidic linkage between C1-of one unit of glucose and C4 of another glucose unit. Thus cellulose contains 1 → 4β glycosidic linkages like those in cellobiose.
Question 36.
Explain the structure of glycogen.
Answer:
The glucose is stored in animal body in the form of glycogen. It is also known as animal starch because its structure is similar to amylopectin. Glycogen is highly branched. Whenever the body is required glucose, enzymes breaks the glycogen to glucose.
![]()
Question 37.
How is glycogen different from starch?
Answer:
Starch is the main storage molecules of plants whereas glycogen is the main storage molecule of animals. Starch is found in cereals, roots, tubers, etc. Glycogen is present in liver, muscles and brain.
Question 38.
What do you understand by the term glycosidic linkage?
Answer:
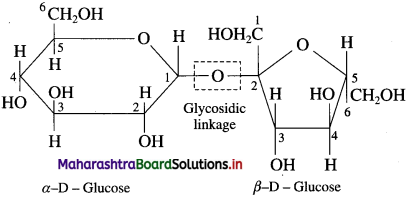
The linkage between two monosaccharide units through oxygen atom is called glycosidic linkage.
Question 39.
What is the basic structural difference between starch and cellulose?
Answer:
Starch is a polymer of a-glucose and consists of two components-amylose and amylopectin. In amylose α-D-D-( + )-glucose units held by C,-C4 glycosidic linkage and in amylopectin, α-D-glucose units held by C1-C4 glycosidic linkage whereas branching αcurs by C1-C6 glycosidic linkage. [Refer Question 35 (i) (ii) Fig.] Cellulose is a straight chain polysaccharide composed only of β-D-glucose units held by C1-C4 glycosidic linkage. (Refer Question 37 Fig.)
Question 40.
Define the term : Protein OR What are proteins?
Answer:
Chemically proteins are polyamides which are high molecular weight polymers of the monomer units i.e. α-amino acids. OR It can also be defined as Proteins are the biopolymers of a large number of a-amino acids and they are naturally occurring polymeric nitrogenous organic compounds containing 16% nitrogen and peptide linkages (-CO-NH-).
Question 41.
Write the common sources of protein.
Answer:
Common sources of proteins are milk, pulses, peanuts, eggs, fishes, cheese, cereals, etc. They are also the principal materials of muscle, nerves, tendons, skin, blood, enzymes, many hormones and antibiotics.
Question 42.
What are the products of hydrolysis of proteins?
Answer:
On hydrolysis, proteins give a mixture of α-anlino acids.
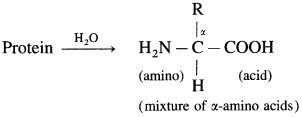
The α-carbon in α-amino acids ohtained by hydrolysis of proteins has ‘L’ configuration.
Question 43.
What are the a-amino acids?
Answer:
α-Amino acids are carboxylic acids having an amino (- NH2) group bonded to the α-carbon, i.e. the carbon next to the carboxyl (- COOH) group.
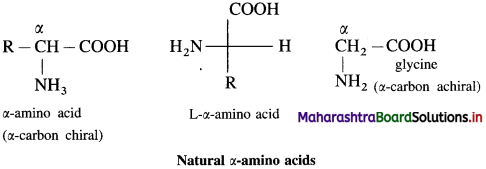
α-amino acids are derivatives of carboxylic acids, obtained by replacing – H atom by amino group. They are bifunctional compounds containing acidic ![]() and basic – NH2 groups.
and basic – NH2 groups.
Example :  (where R is an alkyl group or aryl group).
(where R is an alkyl group or aryl group).
The amino acids are colourless, crystalline, water soluble, high melting solids. These acids in their aqueous solutions behave like salts due to presence of both acidic, ![]() and basic. (- NH2) groups in the same molecule.
and basic. (- NH2) groups in the same molecule.
Such a doubly charged ion is known as zwitter ion. Example : 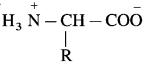
![]()
Question 44.
What are the final products of hydrolysis of proteins?
Answer:
Proteins on hydrolysis with dilute solution of acids, alkalies or enzymes give a mixture of large number of a-amino acids as final products.
For example :
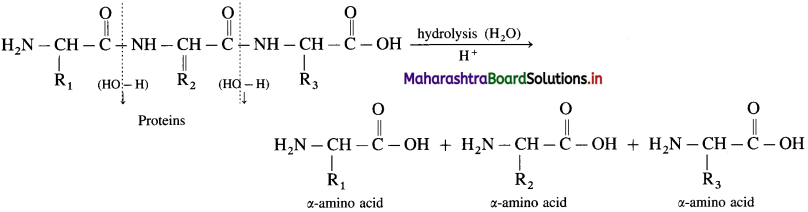
Question 45.
Write the classification of amino acids, giving examples.
Answer:
The amino acids are of three types : acidic, basic and neutral. The symbol ‘R’ in the structure of a-amino acids represents side chain and may contain additional functional groups.
(1) Acidic amino acids : If ‘R’ contains a carboxyl (- COOH) group the amino acid is acidic amino acid, i.e. If carboxyl groups are more in number than amino groups, then amino acids are acidic in nature.
Examples : Glutamic acid HOOC-CH2-CH2-; Aspartic acid HOO-CH2–
(2) Basic amino acids : If ‘R’ contains an amino (1°, 2°, or 3°) group, it is called basic amino acid i.e. If amino groups are more in number than carboxyl groups then amino acids are basic in nature.
Examples : Arginine 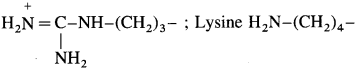
(3) Neutral amino acids : The other amino acids having neutral or no functional group in ‘R’ are called neutral amino acids, i.e. The amino acids having equal number of amino and carboxyl groups are neutral amino acids.
Examples : Alanine CH3-; Valine (CH3)2-CH
Question 46.
What are essential and non-essential amino acids? Give two examples of each.
Answer:
The amino acids, which cannot be synthesised in the body and are supplied through diet are called essential amino acids. Examples : Lysine H2N-(CH2)4-; Valine (CH3)2CH- The amino acids which are synthesized in the body are called non-essential amino acids.
Examples : Glutamic acid HOO-CH2-CH2-; Serine HO-CH2–
Question 47.
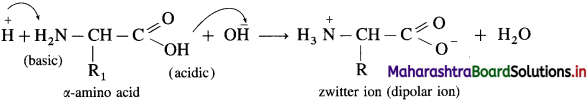
What is meant by Zwitter ion?
Answer:
An a-amino acid molecule contains both acidic carboxyl ( – COOH) group as well as basic amino (- NH2) group. Proton transfer from acidic group to basic group of amino acid forms a salt, which is a dipolar ion called a zwitterion.
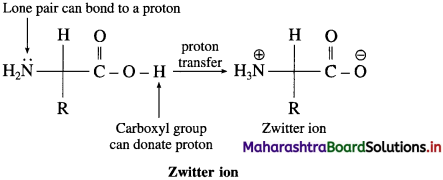
Question 48.
Draw zwitter ion of alanine and other two forms.
Answer:
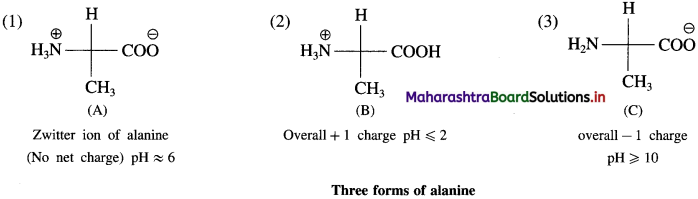
![]()
Question 49.
What is a peptide bond (peptide linkage)?
OR
Define peptide bond.
Answer:
Proteins are the polymers of a-amino acids and they are connected to each other. The bond that connects a-amino acids to each other is called peptide bond (peptide linkage, – CONH -).
Question 50.
How is peptide linkage (dipeptide linkage) formed in proteins? How is tripeptide formed?
Answer:
Peptide linkage is formed by condensation of acidic ![]() group of one molecule of a-amino acid and basic -NH2 group of other molecule of α-amino acid with elimination of water.
group of one molecule of a-amino acid and basic -NH2 group of other molecule of α-amino acid with elimination of water.
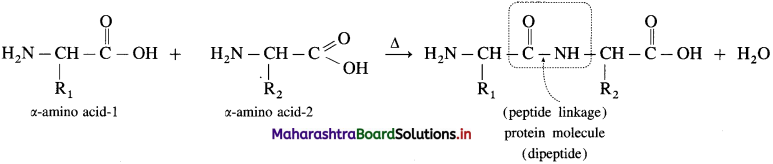
When one more molecule of amino acid combines with dipeptide, it forms tripeptide.
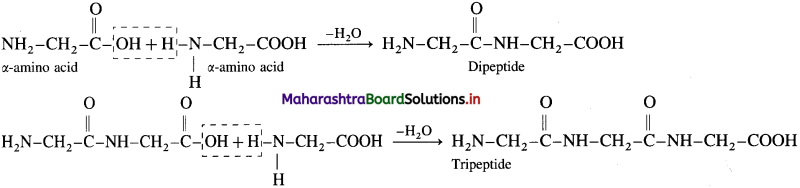
Thus, it forms tetra, penta and finally a polypeptide chain i.e. proteins. Hence, proteins are basically polypeptides.
Question 51.
Write the structures of all possible dipeptides which can be obtained from glycine and alanine.
Answer:
(1) Dipeptide from glycine :
Carboxylic group of glycine reacting with amino group another molecule of glycine to form dipeptide
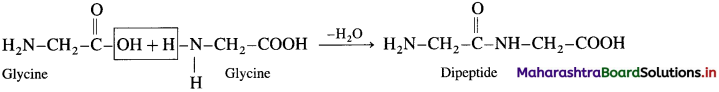
(2) Dipeptide from alanine :
Carboxylic goup of alanine reaction with amino goup of another molecule of alamine to form dipeptide
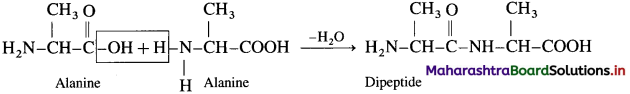
(3) Dipeptide from glycine and alanine :
Carboxylic group of glycine reacting with amino group another molecule of alanine to form dipeptide
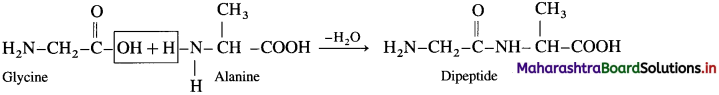
![]()
Question 52.
How are proteins classified on the basis of molecular shapes?
Answer:
On the basis of their molecular shapes proteins are classified as :
(1) Fibrous proteins : The proteins in which the polypeptide chains lie parallel (side by side) to form fibre-like structure, are called fibrous proteins. The polypeptide chains held together by hydrogen bonds. These proteins are insoluble in water.
The fibrous proteins are tough and insoluble in water, and dilute acids or bases.
Example : myαin (in muscles), keratin (in hair, nails, skin), fibroin (in silk), collagen (in tendons), etc.
(2) Globular proteins : The proteins have spherical shape. This shape results from coiling around of the polypeptide chain of protein, and have intramolecular hydrogen bonding are called globular proteins.
They are soluble in water and dilute acids or bases.
Example : Haemoglobin (in blood), albumin (in eggs), insulin (in pancreas), etc.
Question 53.
Distinguish between globular and fibrous proteins.
Answer:
| Globular proteins | Fibrous proteins |
| (1) The chains of polypeptides of protein coil around to give a spherical shape. (2) Globular proteins are soluble in water. (3) They are sensitive to small changes of temperature and pH. (4) They possess biological activity. | (1) The proteins in which the polypeptide chains lie parallel to form fibre like structure. (2) Fibrous proteins are insoluble in water. (3) They are stable to moderate changes of temperature and pH. (4) They do not possess biological activity. |
Question 54.
Draw a neat labelled diagram for the secondary structure of protein.
Answer:
Secondary structure of proteins : The three-dimensional arrangement of lαalized regions of a long polypeptide chain is called the secondary structure of protein. Hydrogen bonding between N-H proton of one amide linkage and C = O oxygen of another gives rise to the secondary structure. There are two different types of secondary structures i.e. α-helix and β-pleated sheet.
α-Helix : In a-helix structure, a polypeptide chain gets coiled by twisting into a right handed or clαkwise spiral known as a-helixn. The characteristic features of α-helical structure of protein are :
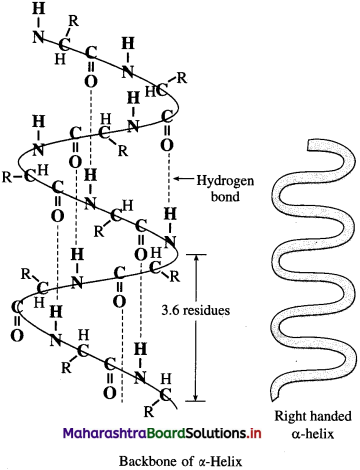
(1) Each turn of the helix has 3.6 amino acids.
(2) A C = O group of one amino acid is hydrogen bonded to N – H group of the fourth amino acid along the chain.
(3) Hydrogen bonds are parallel to the axis of helix while R groups extend outward from the helix core.
Myosin in muscle and a-keratin in hair are proteins with almost entire a-helical secondary structure.
![]()
β-Pleated sheet : In secondary structure, when two or more polypeptide chains (strands) line up side-by-side is called β-pleated sheets. The β-picate sheet structure of protein consists of extended strands of polypeptide chains held together by intermolecular hydrogen bonding. The characteristics of β-pleated sheet structure are :
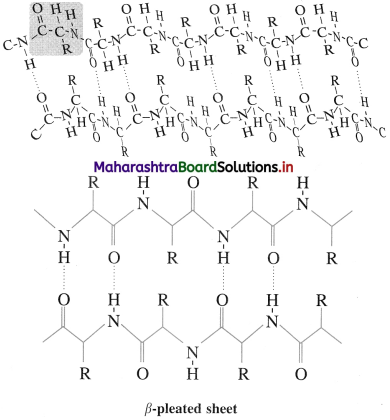
- The C = O and N – H bonds lie in the planes of the sheet.
- Hydrogen bonding occurs between the N – H and C = O groups of nearby amino acid residues in the neighbouring chains.
- The R groups are oriented above and below the plane of the sheet.
The β-pleated sheet arrangement is favoured by amino acids with small R groups.
Question 55.
What is denaturation of proteins? How is denaturation brought about?
OR
What is the effect of denaturation on the structure of proteins?
Answer:
The prαess by which the molecular shape of protein changes without breaking the amide / peptide bonds that form the primary structure is called denaturation. OR Proteins gets easily precipitated. It is an irreversible change and the prαess is called denaturation of proteins.
Denaturation uncoils the protein and destroys the shape and thus loses their characteristic biological activity. Denaturation is brought about by heating the protein with alcohol, concentrated inorganic acids or by salts of heavy metals. During denaturation secondary and tertiary and quternary structures are destroyed but primary structure remains intact.
Example : Boiling of egg to coagulate egg white, conversion of milk to curd.
Question 56.
Define : Enzymes
Answer:
All biological reactions are catalysed by bio-catalyst in living organisms called enzymes.
Question 57.
What are enzymes? Explain with suitable example.
Answer:
All biological or bio-catalysts which catalyse the reactions in living organisms are called enzymes. Chemically all enzymes are proteins. They are required in very small quantities as they are catalyst also they reduce the activation energy for a particular reaction.
Example : Enzyme maltase converts maltose to glucose.
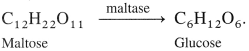
![]()
Question 58.
Explain the catalytic action of enzymes.
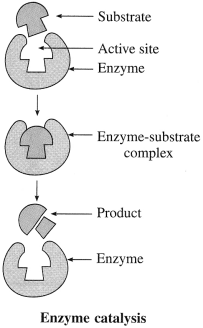
Answer:
Mechanism of enzyme catalysis : Action of an enzyme on a substrate is known as lock-and-key mechanism.
Accordingly, the enzyme has active site on its surface. A substrate molecule can attach to this active site only if it has the right size and shape. Once in the active site, the substrate is held in the correct orientation, enzymes provide functional group which will attack the substrate and forms the products of reaction. The products leave the active site and the enzyme is ready to act as catalyst again.
Question 59.
Give examples of industrial application of enzyme catalysis.
Answer:
- Glucose Isomerase (enzyme) is used in conversion of glucose to sweet-tasting fructose.
- New antibiotics are manufactured using penicillin acylase (enzyme).
- Laundry detergentts are manufactured using proteases (enzyme).
- Esters used in cosmetics are manufactured using genetically engineered enzyme.
Question 60.
Draw a neat diagram for enzyme catalysis.
Answer:

Question 61.
State the main functions of enzymes.
Answer:
Enzymes are biological catalyst and they are highly specific in nature. The two main functions are as follows :
(1) They lower the requirement of activation energy.
(2) They speed up the rate of reaction.
E.g. Enzyme maltase catalyses maltose to glucose.
\(\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11}+\mathrm{H}_{2} \mathrm{O} \stackrel{\text { Maltase }}{\longrightarrow} 2 \mathrm{C}_{6} \mathrm{H}_{12} \mathrm{O}_{6}\)
![]()
Question 62.
What are nucleic acids?
Answer:
Nucleic acids are unbranched polymers of repeating monomers i.e. nucleotides. In other words, nucleic acids have a polynucleotide structure which in turn consists of a base, a pentose sugar and phosphate moiety.
Nucleic acids are biomolecules which are found in the nuclei of all living cells in the form of nucleoproteins or chromosomes.
(Nucleoproteins = Proteins + Nucleic acid)
(prosthetic group)
Question 63.
State the types of nucleic acids.
Answer:
The types of nucleic acids are : Ribonucleic acid (RNA) and deoxy ribonucleic acid (DNA). DNA molecules contain several million nucleotides while RNA molecules contain a few thousand nucleotides.
Question 64.
Explain chemical composition of nucleic acids.
Answer:
Nucleic acids have a polynucleotide structure. Nucleic acids (RNA and DNA) consists of three components :
(1) monosaccharide (sugar)
(2) nitrogen containing base and
(3) phosphate group.
(1) Monosaccharides : Nucleotides of both RNA consist of five membered monosaccharide ring (furanose), called as simply sugar component.
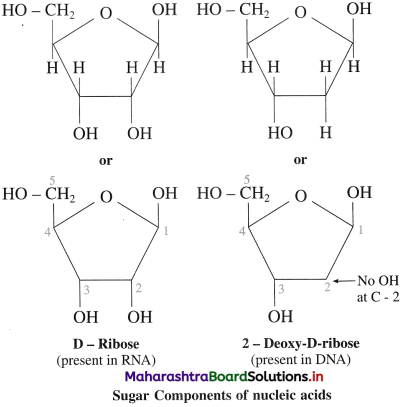
In RNA, the sugar component of nucleotide units is D-ribose and in DNA 2-deoxy-D-ribose.
2 – deoxy means no – OH group at C2 position.
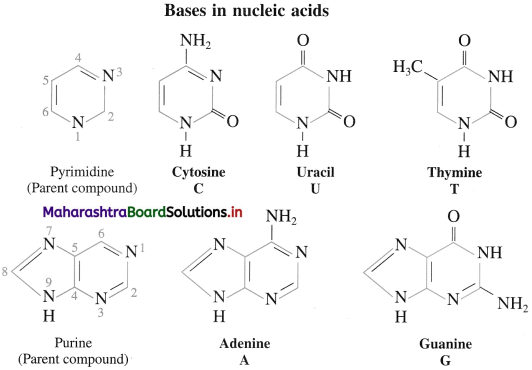
(2) Nitrogen containing base : Total five nitrogen – containing bases are present in nucleic acids. Three bases with one ring (cytosine, uracil and thymine) are derived from the parent compound pyrimidine. Two bases with two rings (adenine and guanine) are derived from the parent compound purine. Each base in designated by a one-letter symbol. Uracil (U) αcurs only in RNA while thymine (T) ocurs only in DNA.
(3) Phosphate group : The sugar units are joined to phosphate through C3 and C5 hydroxyl groups.
![]()
Question 65.
What is meant by nucleosides?
OR
Write the structure of nucleoside. Give examples.
Answer:
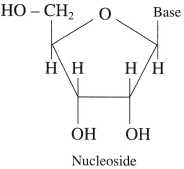
A nucleoside contains two basic components of nucleic acids i.e. a pentose sugar and a nitrogenous base.
A nucleoside is formed when 1 -position of a pyrimidine (cytosine, thymine or uracil) or 9-position of guanine or adenine base is attached to C- l of sugar by β-linkage.
Examples: Formation of nucleoside:
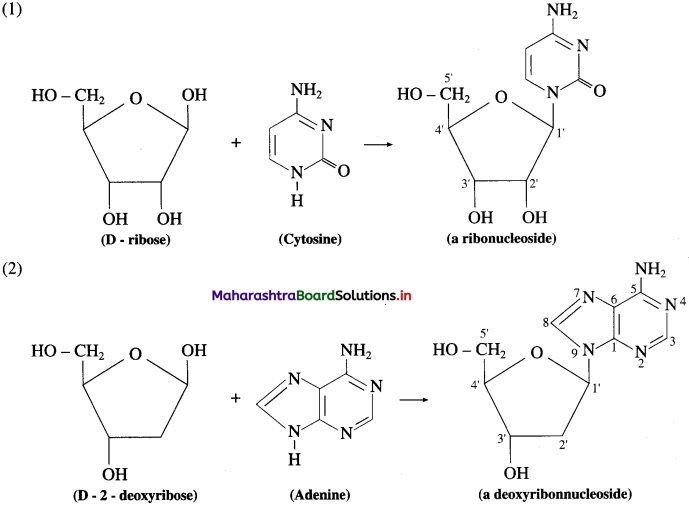
Question 66.
What is meant by nucleotide?
OR
Write the structure of nucleotide. Give example.
Answer:
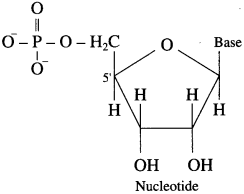
A nucleotide contains all three basic components of nucleic acids i.e., a pentose sugar, a phosphoric acid and a nitrogenous base. These are obtained by esterification of \(\mathrm{C}_{5}^{1}-\mathrm{OH}\) group of the pentose sugar by phosphoric acid. Nucleotides are joined together through phosphate ester linkage. Thus, nucleotides are monophosphates of nucleosides. Abridged names of some nucleotides are AMP, dAMP, UMP, dTMP and so on. Here, the first capital letter is derived from the corresponding base. MP stands for monophosphate. Small letter ‘d’ in the beginning indicates deoxyribose in the nucleotide.
![]()
Example :
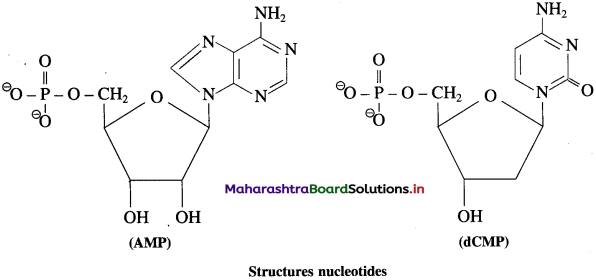
Question 67.
Write the structure of nucleic acids.
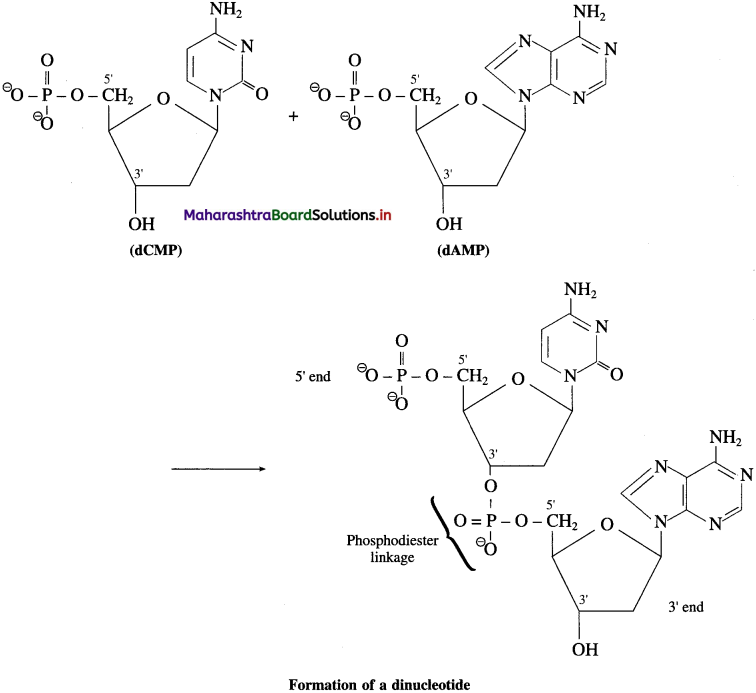
Answer:
Nucleic acids, both DNA and RNA, are polymers of nucleotides, formed by joining the 3′ – OH group of one nucleotide with 5′ – phosphate of another nucleotide. Two ends of polynucleotide chain are distinct from each other. One end having free phosphate group of 5′ position is called 5′ end. The other end is 3′ end and has free OH – group at 3′ position.
Question 68.
Draw a schematic representation of polynucleotide structure of nucleic acids.
Answer:
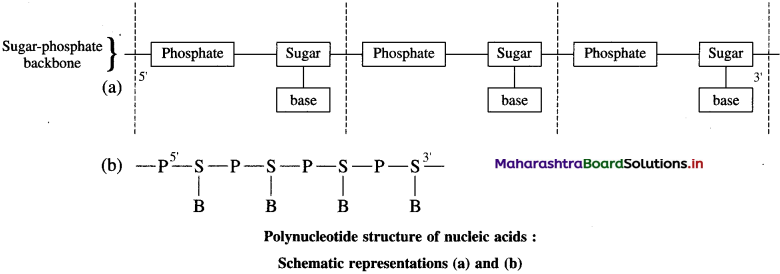
![]()
Question 69.
Explain double helix.
OR
State the salient features of the Watson and Crick mode of DNA.
Answer:
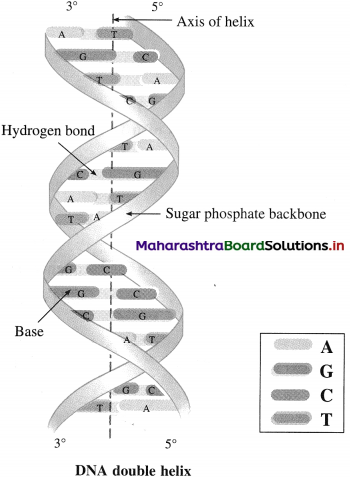
The Salient features are :
- DNA is made of two polynucleotide strands that wind into a right-handed double helix.
- The two strands run in opposite directions: one from the Y end to the 3’ end, while the other from the 3’ end to the Y end.
- Pcrpcndicular to the axis of the helix, the sugar – phosphate backbone lies on the outside of the helix and the bases lic on the inside.
- The hydrogen bonding between the hases of the two DNA strands stabilizes the double helix. This gives rise to a ladder-like structure of DNA double helix.
- Adenine always forms two hydrogen bonds with thymine and guanine forms three hydrogen bonds with cytosinc. Thus A – T arid C – G arc complementary hase pairs and the Two strands of the double helix arc complementary to each other.
Question 70.
Give scientific reasons :
1. In the preparation of glucose from sucrose, ethyl alcohol is added at the time of cooling.
Answer:
Hydrolysis of sucrose with dilute hydrαhloric acid gives glucose along with fructose.
Ethyl alcohol is added at the time of cooling in the preparation of glucose, to separate glucose from fructose. Glucose being insoluble in alcohol, crystallizes out first, while fructose being more soluble in alcohol, remains in the solution.
Question 71.
Answer in one sentence :
(1) How is glucose stored in the animal body?
Answer:
Glucose is stored in the form of glycogen in the animal body.
(2) Write other term used for carbohydrates.
Answer:
Carbohydrates are often termed as saccharides or sugars.
(3) How many moles of acetic anhydride will be required to form glucose penta acetate from 1 mole of glucose?
Answer:
10 moles of acetic anhydride.
(4) What are reducing sugars?
Answer:
Reducing sugars : Carbohydrates which reduce Fehling solution to red ppt of Cu20 or Tollen’s reagent to shining metallic silver are called reducing sugars. All monosaccharides and oligosaccharides except sucrose are reducing sugars.
![]()
(5) What are non-reducing sugars?
Answer:
Non-reducing sugars : Carbohydrates which do not reduce Fehling solution and Tollen’s reagent are called non-reducing sugars. E.g. sucrose.
(6) Give an example each of reducing and non-reducing sugars.
Answer:
Reducing sugars : Maltose or lactose
Non-reducing sugars : Sucrose.
(7) Name the linkage which joins two monosaccharide units through oxygen atom.
Answer:
The linkage which joins two monosaccharide units through oxygen atom is called glycosidic linkage.
(8) Name the sugar present in DNA.
Answer:
The sugar present in DNA is deoxyribose.
(9) A nucleotide from DNA containing thymine is hydrolysed. What are the products formed?
Answer:
When nucleotide from DNA containing thymine is hydrolysed, 2-deoxy-D-ribose, thymine and phosphoric acid is obtained.
(10) How is zwitterion formed?
Answer:
In aqueous solution, the carboxyl group loses a proton while the amino group accepts it, as a result, a dipolar or zwitter ion is formed.
(11) Name the amino acids which are synthesized in the body.
Answer:
The amino acids which are synthesized in the body are called non-essential amino acids. Examples : Glutamic acid, serine.
(12) Name the four bases present in DNA which of these is not present in RNA.
Answer:
Purines-adenine (A) and guanine (G); Pyrimidines-thymine (T) and cytosine (C), these four bases are present in DNA. Out of these, thymine (T) is not present in RNA.
(13) What are different types of RNA which are found in the cell?
Answer:
There are three different types of RNA found in the cell. (1) The messenger RNA which carries the message to the ribosome (2) Ribosomal RNA where synthesis of protein takes place (3) The transport RNA.
(14) State the functions of RNA and DNA.
Answer:
RNA and DNA are responsible for generic characteristics : DNA preserves the information and uses it by producing duplicate identical DNA molecules. RNA carries messages and transports them.
Multiple Choice Questions
Question 72.
Select and write the most appropriate answer from the given alternatives for each subquestion :
1. Which of the following is not sugar?
(a) Sucrose
(b) Starch
(c) Fructose
(d) Glucose
Answer:
(b) Starch
2. Which of the following is the example of disaccharide?
(a) Glucose
(b) Raffinose
(c) Cellulose
(d) Sucrose
Answer:
(d) Sucrose
3. Fructose is
(a) aldopentose
(b) aldohexose
(c) ketopentose
(d) ketohexose
Answer:
(d) ketohexose
4. Oxidation product of glucose with bromine water is
(a) sorbitol
(b) gluconic acid
(c) glutamic acid
(d) saccharic acid
Answer:
(b) gluconic acid
![]()
5. The general formula of carbohydrates is
(a) C(H2O)
(b) Cx(H2O)y
(c) Cx(H2O)
(d) Cx(H2O)x
Answer:
(b) Cx(H2O)y
6. Monosaccharides containing aldehyde group are called
(a) aldoses
(b) ketoses
(c) polysaccharides
(d) disaccharides
Answer:
(a) aldoses
7. Which of the following sugars can be used to prepare glucose on a large scale?
(a) Cellulose
(b) Cane sugar
(c) Galactose
(d) Starch
Answer:
(d) Starch
8. Which of the following carbohydrates cannot undergo hydrolysis?
(a) Glucose
(b) Sucrose
(c) Cellulose
(d) Maltose
Answer:
(a) Glucose
9. Glucose differs from fructose in
(a) the functional group
(b) the number of chiral carbon atoms
(c) the number of carbon atoms
(d) both (a) and (b)
Answer:
(d) both (a) and (b)
10. The example of aldopentose is
(a) arabinose
(b) glucose
(c) fructose
(d) sucrose
Answer:
(a) arabinose
11. Dextrose, grape sugar and blood sugar αcurs in
(a) fructose
(b) glucose
(c) sucrose
(d) starch
Answer:
(b) glucose
12. The example of ketopentose is
(a) galactose
(b) ribose
(c) raffinose
(d) maltose
Answer:
(b) ribose
![]()
13. Cane sugar on hydrolysis gives
(a) glucose and maltose
(b) glucose and lactose
(c) glucose and fructose
(d) only glucose
Answer:
(c) glucose and fructose
14. On commerical scale, glucose is prepared from
(a) starch
(b) potato pulp
(c) sucrose
(d) both (a) and (b)
Answer:
(d) both (a) and (b)
15. The number of monosaccharide units formed on hydrolysis of glucose are
(a) zero
(b) one
(c) two
(d) three
Answer:
(a) zero
16. Which of the following is NOT TRUE about glucose?
(a) It is monosaccharide
(b) It is a polyhydroxy aldehyde
(c) It is polyhydroxy ketone
(d) It contains six carbon atoms
Answer:
(c) It is polyhydroxy ketone
17. Final hydrolysis product of simple protein is
(a) carboxylic acid
(b) α-amino acid
(c) mineral acid
(d) acetic acid
Answer:
(b) α-amino acid
18. Haemoglobin is the example of-
(a) simple protein
(b) derived protein
(c) fibrous protein
(d) conjugated protein
Answer:
(d) conjugated protein
19. Protein are also called
(a) polysaccharides
(b) polypeptides
(c) polyglycerides
(d) polyster
Answer:
(b) polypeptides
20. The simplest amino acid is
(a) glycine
(b) oxalic acid
(c) adipic acid
(d) caprolactam
Answer:
(a) glycine
![]()
21. Amino acids usually exist in the form of zwitter ion which consist of
(a) the basic group-NH2 and the acidic group -COOH
(b) the acidic group -N+H3 and the basic group COO–
(c) the acidic group -COO+ and the acidic group NH3-
(d) acidic or basic group
Answer:
(b) the acidic group -N+H3 and the basic group COO-
22. The water insoluble protein is
(a) casein of milk
(b) albumin
(c) serum albumin
(d) keratin of hair
Answer:
(d) keratin of hair
23. The main structural feature of a protein molecule is the presence of
(a) an ester linkage
(b) an ether linkage
(c) a peptide linkage
(d) all of these
Answer:
(c) a peptide linkage
24. Milk sugar is
(a) sucrose
(b) lactose
(c) maltose
(d) glucose
Answer:
(b) lactose
25. The carbohydrates used for silvering of mirror is
(a) fructose
(b) starch
(c) glucose
(d) cellulose
Answer:
(c) glucose
26. Which one of the following is NOT produced by human body?
(a) DNA
(b) Hormones
(c) Enzymes
(d) Vitamins
Answer:
(c) Enzymes
27. A biological catalyst is essentially
(a) an amino acid
(b) an enzyme
(c) a nitrogen molecule
(d) a carbohydrate
Answer:
(d) a carbohydrate
28. Which one of the following is not a constituent of RNA?
(a) Ribose
(b) Uracil
(c) Thymine
(d) Phosphate
Answer:
(b) Uracil
![]()
29. DNA is a polymer of units of
(a) sugars
(b) ribose
(c) amino acids
(d) nucleotides
Answer:
(c) amino acids
30. Which one of the following molecules will form zwitter ion?
(a) CH3COOH
(b) CH3CH2NH2
(c) CCl3NO2
(d) NH2CH2COOH
Answer:
(d) NH2CH2COOH
31. In metabolic prαess the maximum energy is given by
(a) carbohydrates
(b) proteins
(c) vitamins
(d) fats
Answer:
(d) fats
32. DNA has a structure of helix was reported by
(a) Herman Fischer
(b) Fedrick Sauger
(c) Andreas Marggraf
(d) Watson and Crick
Answer:
(d) Watson and Crick
33. The secondary structure of a protein is determined by
(a) co-ordinate bond
(b) covalent bond
(c) ionic bond
(d) hydrogen bond
Answer:
(d) hydrogen bond
34. In maltose, glycosidic linkage is present between the two glucose units at positions
(a) 1, 2
(b) 1, 1
(c) 1, 3
(d) 1, 4
Answer:
(d) 1, 4
35. Which of the following amino acids is basic in nature?
(a) Valine
(b) Tyrosine
(c) Arginine
(d) Luecine
Answer:
(c) Arginine
![]()
36. Sucrose molecules consists of
(a) a glucofuranose and a fructopyranose
(b) a glucofuranose and a fructofuranose
(c) a glucopyranose and a fructopyranose
(d) a glucopyranose and a’ fructofuranose
Answer:
(d) a glucopyranose and a’ fructofuranose
37. Which one of the following statements is not correct about DNA molecule?
(a) It has double helix structure
(b) It serves as hereditary material
(c) The two DNA strands are exactly similar
(d) Its replication is called semi-conservative mode of replication
Answer:
(c) The two DNA strands are exactly similar
38. Glycine on heating forms
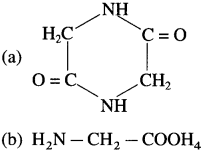
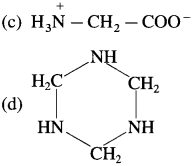
Answer:
(a)
39. Acidic amino acid is
(a) Glutamine
(b) Glutamic acid
(c) Tyrosine
(d) Lysine
Answer:
(b) Glutamic acid
40. Basic amino acid is
(a) Lysine
(b) Glycine
(c) Cystine
(d) Alanine
Answer:
(a) Lysine
41. Precipitation of protein is referred to as
(a) destruction of proteins
(b) separation of proteins
(c) denaturation of proteins
(d) fragmentation of proteins
Answer:
(c) denaturation of proteins
42. An amino acid containing sulphur is
(a) serine
(b) cysteine
(c) valine
(d) asparagine
Answer:
(b) cysteine
![]()
43. Rhamnose is a
(a) carbohydrate
(b) protein
(c) lipid
(d) vitamin
Answer:
(a) carbohydrate
44. Lactose on hydrolysis gives
(a) glucose + glucose
(b) glucose + fructose
(c) glucose + galactose
(d) fructose + galactose
Answer:
(c) glucose + galactose
45. Raffinose on hydrolysis gives
(a) glucose + glucose + galactose
(b) glucose + fructose + galactose
(c) glucose + galactose + galactose
(d) fructose + galactose + galactose
Answer:
(b) glucose + fructose + galactose
46. Naturally αcurring glucose is
(a) dextro rotatory
(b) laevo rotatory
(c) racemic mixture
(d) all of these
Answer:
(a) dextro rotatory
47. Amylopectin is
(a) soluble in water and constitutes about 80% of starch
(b) insoluble in water and constitutes about 80% of starch
(c) Soluble in alcohol and constitutes about 60% of starch
(d) in soluble in alcohol and constitutes about 60% of starch
Answer:
(b) insoluble in water and constitutes about 80% of starch
48. Insulin contains
(a) 51 amino acids
(b) 151 amino acids
(c) 15 amino acids
(d) 115 amino acids
Answer:
(a) 51 amino acids
49. Pyranose structure of glucose is
(a) an open chain structure of glucose
(b) a structure of reduction product of glucose
(c) a cyclic six-membered structure of glucose
(d) a four-membered cyclic form of glucose
Answer:
(c) a cyclic six-membered structure of glucose
50. The number of – OH groups present in ribulose is
(a) 3
(b) 4
(c) 6
(d) 5
Answer:
(b) 4
![]()
51. Peptide linkage is
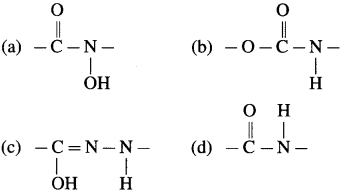
Answer:
(d)
52. Stachyose is an example of
(a) monosaccharides
(b) disaccharides
(c) trisaccharides
(d) tetrasaccharides
Answer:
(d) tetrasaccharides
53. How many moles of (CH3CO)2O will be required to form glucose pentaacetate form 2 moles of glucose?
(a) 2
(b) 5
(c) 10
(d) 2.5
Answer:
(c) 10
54. Which of the following NOT present in DNA?
(a) Adenine
(b) Guanine
(c) Thymine
(d) Uracil
Answer:
(d) Uracil
![]()
55. Maltose is a
(a) polysaccharide
(b) disaccharide
(c) trisaccharide
(d) monosaccharide
Answer:
(b) disaccharide