Balbharti Maharashtra State Board 12th Chemistry Important Questions Chapter 13 Amines Important Questions and Answers.
Maharashtra State Board 12th Chemistry Important Questions Chapter 13 Amines
Question 1.
What are amines?
Answer:
Amines : The alkyl or aryl derivatives of ammonia in which one, two or all the three hydrogen atoms attached to nitrogen are replaced by same or different alkyl or aryl groups are called amines. OR Amines are nitrogen-containing organic compounds having basic character.
Example : methyl amine : CH3 – NH2
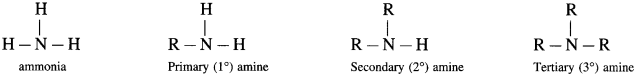
![]()
Question 2.
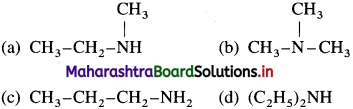
Classify the following amines as primary, secondary and tertiary.
Answer:

Question 3.
Mention the functional group in :
(1) Primary amine
(2) Secondary amine
(3) Tertiary amine.
Answer:
(1) A primary amine has a functional group – NH2 (amino group).
Example : ethylamine, C2H5 – NH2
(2) A secondary amine has a functional group – NH – (imino group).
Example : 
(3) A tertiary amine has a functional group ![]() (tertiary nitrogen atom)
(tertiary nitrogen atom)
Example :

Question 4.
Write common and IUPAC names of following compounds :
Answer:
(A) Primary amines :

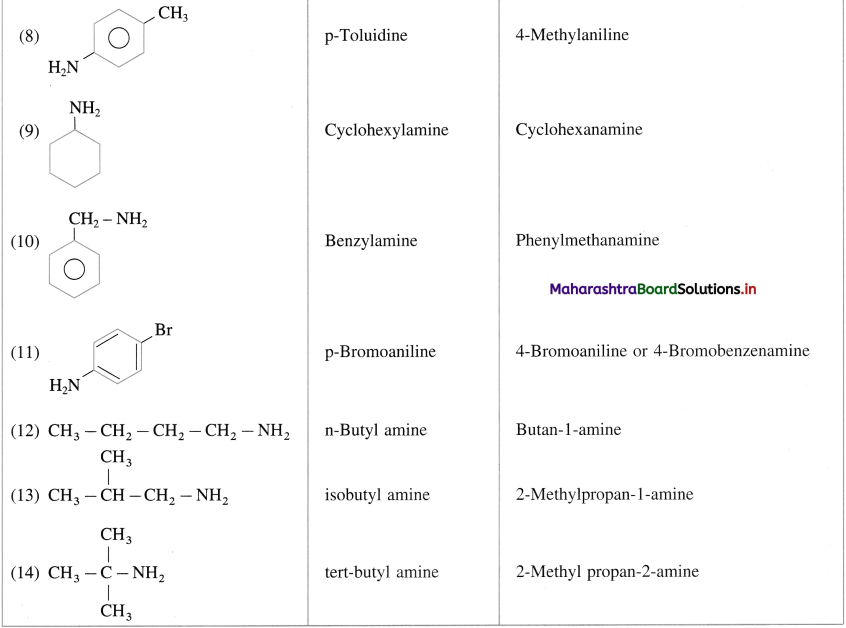
(B) Secondary amine :

(C) Tertiary Aimines :
 .
.
![]()
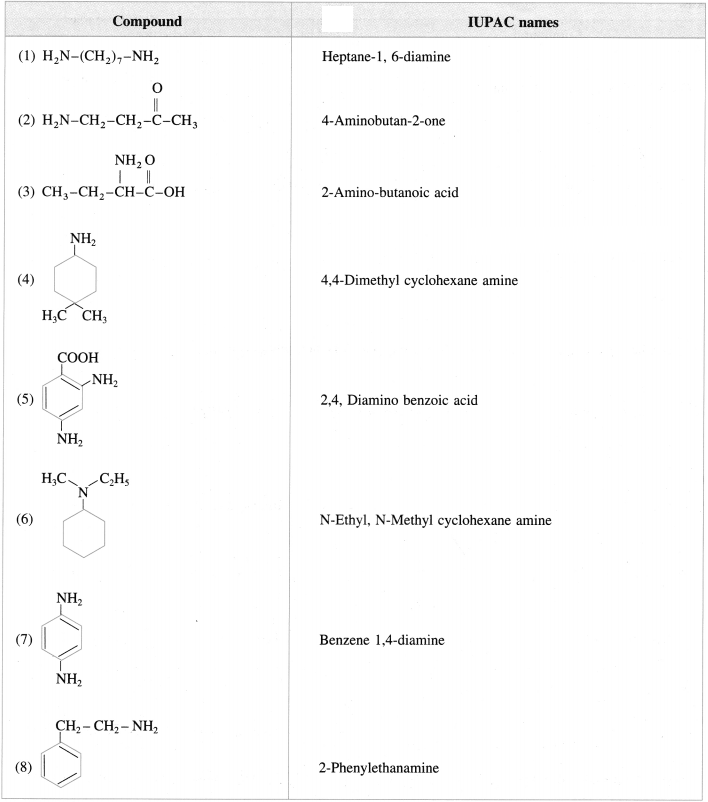
Question 5.
Give the structures of the following :
Answer:

Question 6.
Give the IUPAC names of the following amines :
Answer:

Question 7.
Write the IUPAC names of the following amines :
Answer:
![]()
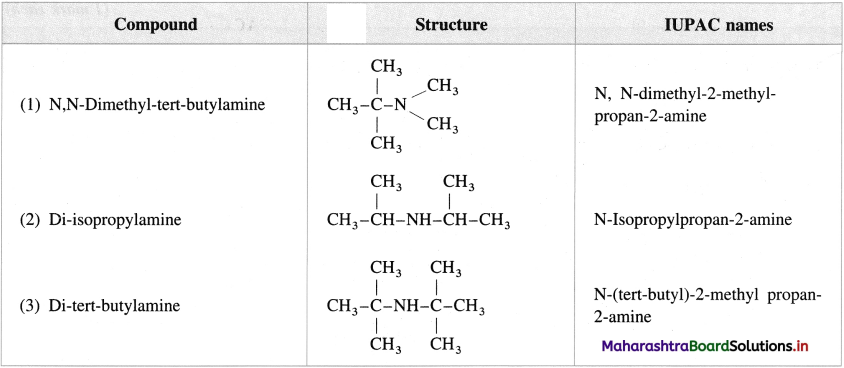
Question 8.
Give the structures and IUPAC names of the following amines :
Answer:
Question 9.
Classify the following amines as primary, secondary and tertiary and write the IUPAC names.
Answer:
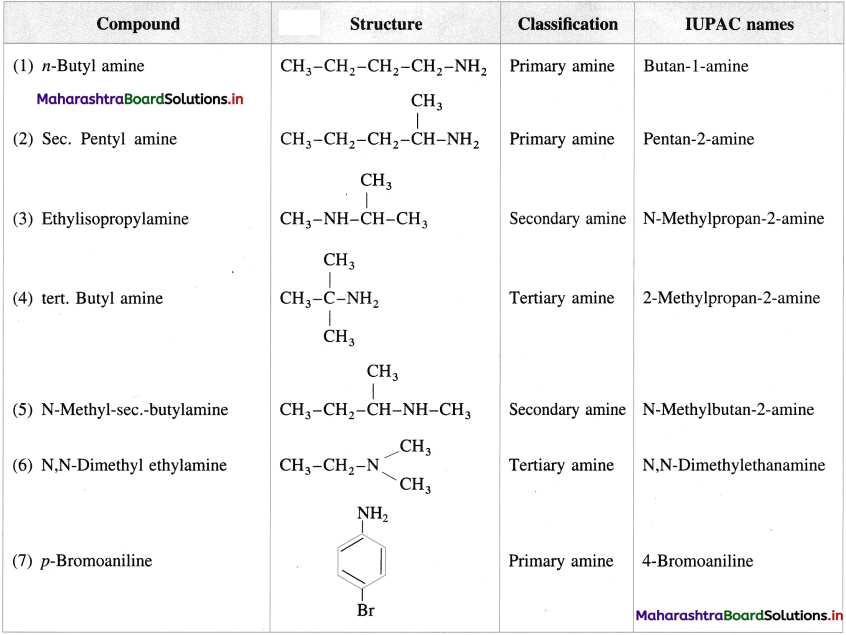
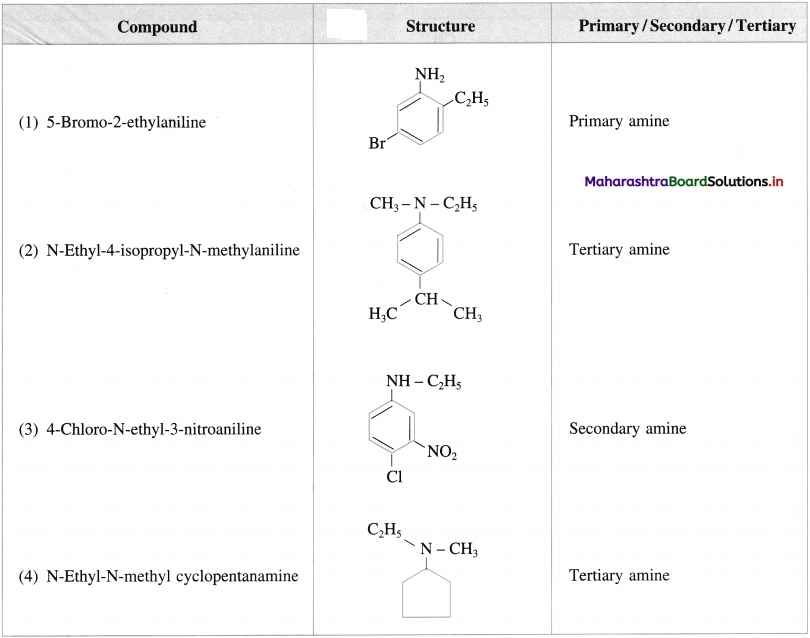
Question 10.
Write the structures and classify the following amines as primary, secondary, tertiary amines.
Answer:
![]()
Question 11.
Write the common and IUPAC name of a tertiary amine in which one methyl, one ethyl and one w-propyl group is attached to nitrogen.
Answer:

Question 12.
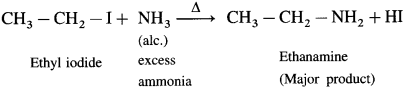
How will you prepare ethanamine from ethyl iodide?
Answer:
When ethyl iodide is heated with excess of alcoholic ammonia, under pressure at 373 K ethanamine is obtained as a major product.
Question 13.
How is a nitroalkane converted to a primary amine?
OR
What is the action of LiAlH4/ether on (i) 1-Nitropropane (ii) 2-MethyI-l-nitropropane?
Answer:
When a nitroalkane is refluxed with tin (or iron) and concentrated HCl it gives corresponding primary amine.
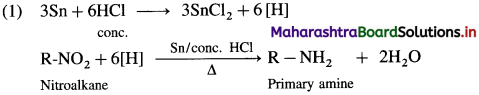
For example, (1) nitromethane on reduction by refluxing with Sn and concentrated HCl gives methylamine.
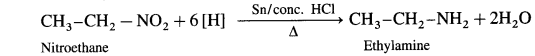
(2) 1-Nitropropane on reduction with Sn and concentrated HCl gives propan-1-amine.
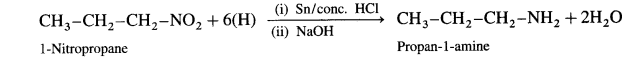
(3) Niirobenzcnc on reducion with tin and concentrated HCI or by using H2/Pd in ethanol gives anilinc.

(4) When nitropropane is reduced in the presence of LiAlH4 in ether, n-propyl amine is obtained.

(5) When 2-methyl-1-nitropropane is reduced in the presence of LiAlH4 in ether, 2-methyl propan-1-amine is obtained.

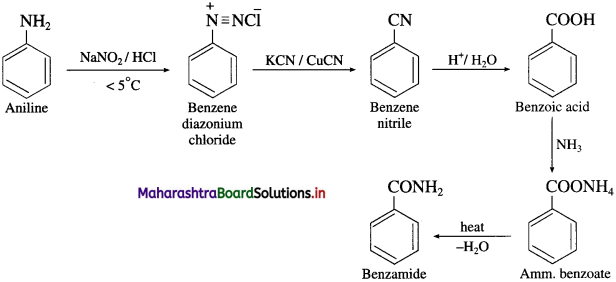
Question 14.
How will you prepare aniline from nitrobenzene?
OR
How is aniline prepared from nitro compounds?
Answer:
Nitrobenzene is reduced to aniline by passing hydrogen gas in the presence of finely divided nickel, palladium or platinum.

Question 15.
Identify the compounds A and B in the following reactions
![]()
Answer:
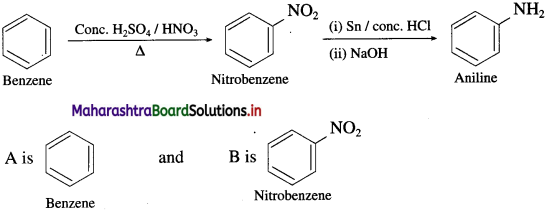
![]()
Question 16.
How will you obtain a primary amine from an alkyl cyanide (nitrile)?
OR
Write a short note on Mendius reduction.
Answer:
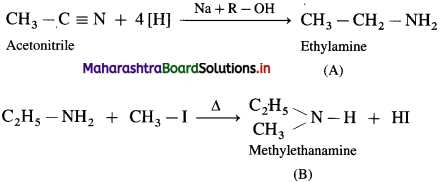
Alkyl cyanides (nitriles) on reduction by sodium and ethyl alcohol form corresponding primary amines. This reaction is called Mendius reduction.
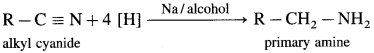
For example; propionitrile on reduction by sodium and ethanol gives n-propyl amine (Propan-1-amine).
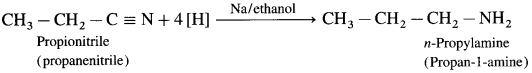
Methyl cyanide or acetonitrile on reduction by sodium and ethanol gives ethanaminc.
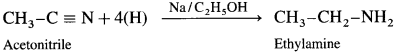
Question 17.
How will you prepare ethylamine from acetonitrile?
OR
How is ethanamine prepared from methyl cyanide?
OR
What is the action of a mixture of sodium and alcohol on acetonitrile?
Answer:
Methyl cyanide or acetonitrile on reduction by sodium and ethyl alcohol forms ethanamine. The reaction is called Mendius reduction.

Question 18.
How will convert phenyl acetonitrile to β-phenylethylamine?
Answer:
When phenyl acetonitrile is reduced in the presence of sodium and ethanol, β-phenyl ethylamine is obtained.

Question 19.
How will you obtain primary amine from an acid amide?
Answer:
Acid amides on reduction with lithium aluminium hydride or sodium, ethanol form corresponding primary amines.

For example : Acetamide on reduction with lithium aluminium hydride or sodium, ethanol gives ethylamines.

Question 20.
Explain Hoffmann degradation of amides.
Write a note on Hoffmann bromamide degradation.
Answer:
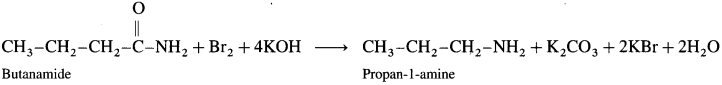
The conversion of amides into amines in the presence of bromine and alkali is known as Hoffmann degradation of amides. An important characteristic of this reaction is that an amine with one carbon less than those in the amide is formed. Thus, decreasing the length of carbon chain. This reaction is an example of molecular rearrangement and involves the migration of an alkyl or aryl group from the carbonyl carbon to the adjacent nitrogen atom. For example,
(1) When propanamide is treated with bromine and aqueous or alcoholic sodium hydroxide, ethanamine is obtained which has one carbon atom less.

(2) When benzamide is treated with bromine and aqueous or alcoholic sodium hydroxide, aniline is obtained.
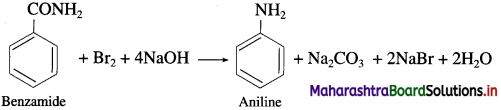
![]()
Question 21.
How will you obtain methyl amine from acetamide?
Answer:
When acetamide is treated with bromine and aq or alcoholic solution of KOH, methyl amine is obtained, which has one cabon atom less.

Question 22.
How will you convert the following?
(1) Ethyl bromide to ethylamine.
Answer:

(2) Propionitrile to n-propyl amine.
Answer:

(3) Acetonitrile to ethylamine.
Answer:

(4) Phenyl acetonitrile to β-phenylethyl amine.
Answer:

(5) Acetamide to ethylamine.
Answer:
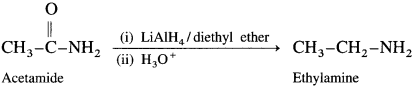
(6) Nitropropane to propan-l-amine.
Answer:
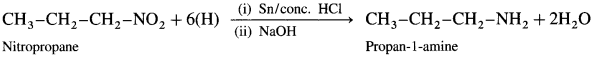
(7) Nitrobenzene to Aniline.
Answer:

(8) Benzamide to aniline.
Answer:
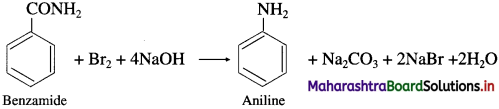
![]()
Question 23.
How will you prepare propan-l-amine from (1) butane nitrile (2) 1-nitropropane (3) propanamide (4) butanamide?
Answer:
(1) From butane nitrile :
When butane nitrile is reduced by sodium and ethanol, it gives propan-l-amine.

(2) From 1-nitropropane :
When 1-nitropropane is reduced in the presence of tin and cone, hydrochloric acid, propan-l-amine is obtained.
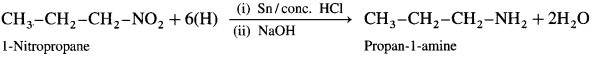
(3) From propanamide :
When propanamide is reduced in the presence of lithium aluminium hydride, propan-l-amine is obtained.
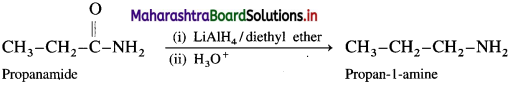
(4) From butanamide :
When butanamide is treated with bromine and aq. KOH, propan-l-amine is obtained.
Question 24.
Write a reaction to, convert acetic acid into methyl amine.
Answer:

Question 25.
Primary and secondary amines have boiling points higher than the tertiary amines. Explain why?
Answer:
(1) The N – H bond in amines is polar in nature because of electronegativities of nitrogen (3.0) and hydrogen (2.1) are different.
(2) Due to the polar nature of N – H bond, primary and secondary have strong intermolecular hydrogen bonding. Tertiary amines do not have intermolecular hydrogen bonding as there is no hydrogen atom on nitrogen of tertiary amine. Thus, intermolecular forces of attraction are strongest in primary and secondary amines and weakest in to tertiary amines. Hence, primary and secondary amines have boiling points higher than the tertiary amines.
Question 26.
Amines have boiling points higher than the hydrocarbon but lower than the alcohols of comparable masses. Explain, why?
Answer:
Amines are polar than alkanes but less polar than alcohols. Primary and secondary amines form intermolecular hydrogen bonds. This hydrogen bonding leads to an associated structure. The association is more in primary amines than that in secondary amines as there are two hydrogen atoms attached to the nitrogen atom. However, tertiary amines do not form intermolecular hydrogen bonds because they do not contain any hydrogen atoms attached to the nitrogen atom. Hence, amines have higher boiling points than the hydrocarbons but lower boiling points than the alcohols of comparable masses.

| Compound | Molar mass | Boiling points (K) |
| nC2H5CH(CH3)2 | 72 | 300 |
| nC4H9NH2 | 73 | 350.8 |
| nC4H9OH | 74 | 391 |
Question 27.
Arrange the following compounds in the decreasing order of their solubility in water.
(a) Ethyl amine, diethyl amine and triethyl amine.
Answer:
Diethyl amine > triethyl amine > ethyl amine
(The reason that ethyl group has greater +1 effect than methyl group)
![]()
(b) Ethyl amine, n-propyl amine and n-butyl amine.
Answer: n-butyl amine < n-propyl amine < ethyl amine
(c) n-Butane, n -butyl alcohol and n-butyl amine
Answer:
n-butyl alcohol < n-butyl amine < n-butane
Question 28.
Arrange the following compounds in the decreasing order of their boiling points.
(a) Ethane, ethyl amine and ethyl alcohol.
Answer:
Ethyl alcohol < ethyl amine < ethane
(b) Ethyl amine, n-propyl amine and n-butyl amine.
Answer:
n-butyl amine < n-propyl amine < ethyl amine
(c) n-propyl amine, ethyl methyl amine and trimethyl amine.
Answer:
n-propyl amine < ethyl methyl amine < trimethyl amine.
(d) Ethyl alcohol, dimethyl amine and ethyl amine.
Answer:
Ethyl alcohol < ethyl amine < dimethyl amine.
Question 29.
Explain the basic nature of amines with a suitable examples.
OR
Explain why amines are basic.
Question 38.
Tertiary amine (R3N) or 3° amine is weaker base than secondary amine R2NH or 2° amine. Explain.
Answer:

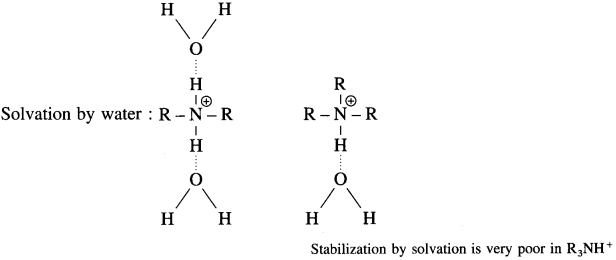
The increase in basic strength from 1° amine to 2° amine is explained on the basis of increased stabilization of conjugate acids by +1 effect of the increased number of the alkyl group. However, decreased basic strength of 3° implies that the conjugate acid of 3° amine is less stabilized and is weak base though the +1 effect of three alkyl groups in R3NH is large.
R2NH is best stabilized by solvation while the stabilization by solvation is very poor in R3NH. Hence (R3N) or tertiary amine or 3° amine is weaker base than secondary amine (R2NH) or 2° amine.
Question 30.
Primary or aliphatic amine is a stronger base than ammonia. Explain.
Answer:
(1) The alkyl group in primary amines has +I effect i.e. (electron releasing).
![]()
The alkyl group tends to increase the electron density on the nitrogen atom. As a result, amines can donate the lone pair of electrons on nitrogen more easily than ammonia.
(2) The amine being a base, can donate a pair of electrons to an acid. The alkyl group with +I effect will disperse the positive charge on the cation more than ammonia.

Due to +I effect of alkyl group cation formed by primary amine is more stable compared to cation formed from ammonia. Also it is seen that observed increasing basic strength from ammonia to primary amine is explained on the basis of increased stabilization of conjugate acids by +I effect for the presence of alkyl (R) groups. Hence, primary or aliphatic amine is a stronger base than ammonia.
![]()
Question 31.
Aniline is less basic than ammonia. Explain.
Answer:
The less basic character of aniline can be explained on the basis of resonance shown by aniline.

Due to resonance, the nitrogen atom of amino group in aniline acquires a positive charge, hence, lone pair of electrons is less available for protonation as compared to that of ammonia. Aniline is resonance stabilized by five resonance structures. On the other hand, aniline in aqueous medium, accepts a proton does not have lone pair of electrons on nitrogen to produce a very low concentration of anilium ion and anilium ion shows only two resonance structures and therefore less stabilized than anline.
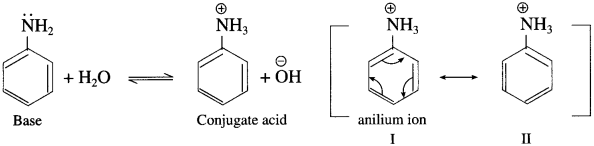
Thus, aniline is more stable than anilium ion. Hence aniline accepts proton less readily or less basic in nature than ammonia.
Question 32.
Explain the order of basicity in ammonia and aliphatic amines.
Answer:
Since nitrogen atom in ammonia molecule has a lone pair of electrons, it is a Lewis base.
Greater the availability of an electron pair, more is the basic character.
Since alkyl group (R -) is an electron releasing group with (+I) inductive effect, alkyl amines act as a stronger base than ammonia.
The decreasing order of basicity is –

The availability of a lone pair of electrons on a nitrogen atom in amines is influenced by steric factor due to crowding of alkyl groups which affects solvation along with inductive effect of alkyl groups.
Due to high energy of solvation of \(\mathrm{NH}_{4}^{+}\) ions, they acquire higher stability in aqueous solutions.
The presence of alkyl groups in secondary and tertiary amines, due to steric hindrance decrease the solvation energy.
This effect is more in tertiary amines making the tertiary ammonium ions (R3NH+) unstable as compared to secondary ammonium ion (R2N+H2).
Hence the cumulative effect on the order of basicity of amines is, secondary amine > primary amine > tertiary amine > ammonia (NH3).
Question 33.
Arrange the following amines in the decreasing order of their basic nature.
(a) Aniline, propan-l-amine and N-methylethanamine.
Answer:
N-methylethanamine < propan-l-amine < aniline
(b) Benzene-1, 4-diamine, ammonia and 4-aminobenzoic acid.
Answer:
Ammonia < benzene-1, 4-diamine < 4-aminobenzoic acid
(c) N-Methylaniline, phenylmethylamine and N-phenylaniline.
Answer:
N-Methylaniline < N-phenylaniline < phenylmethylamine
Question 34.
Arrange the following amines in the increasing order of their pKb values.
(a) Aniline, N-methylaniline and cyclohexalamine.
Answer:
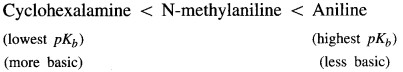
![]()
(b) Phenylmethylamine, 2-aminotoluene and 2-fluoroaniline.
Answer:

(c) Aniline, 4-methoxyaniline and 4-nitroaniline.
Answer:

Question 35.
Arrange the following compounds in the decreasing order of their basic nature in the gaseous phase.
Ammonia, N-methylhexanamine, propan-1-amine and N, N-dimethylethanolamine.
Answer:
Propan-1-amine < N-methylethanamine < N,N-dimethylmethanamine < ammonia
Question 36.
Explain laboratory test for amines.
Answer:
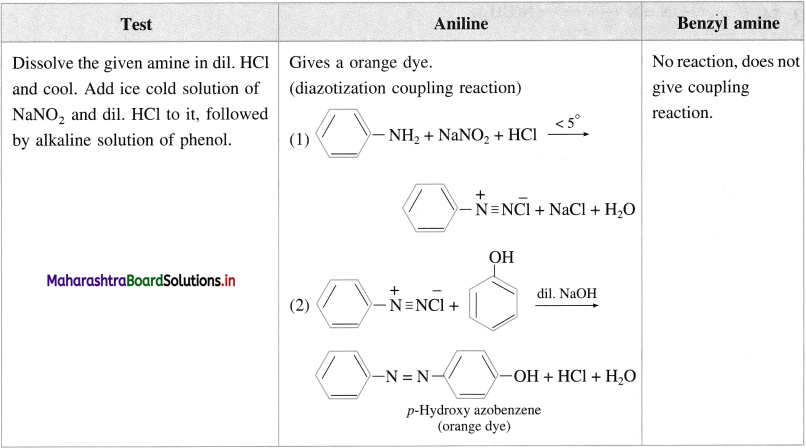
(1) All amines are basic compounds. Aqueous solution of water soluble amines turns red litmus blue.
(2) When water insoluble amine is dissolved in aqueous HCl, forms water soluble substituted ammonium chloride, further a substituted ammonium chloride on reaction with excess aqueous NaOH regenerates the original insoluble amine.

(3) Diazotization reaction/ Orange dye test: In a sample of aromatic primary amine, 1-2 mL of cone. HCl is added. The aqueous solution of NaNO2 is added with cooling. This solution is transfered to a test tube containing solution of β naphthol in NaOH. Formation of orange dye indicates presence of aromatic primary amino group. (It may be noted that temperature of all the solutions and reaction mixtures is maintained near 0 °C throughout the reaction).
Question 37.
Explain Hofmann’s exhaustive alkylation.
OR
Explain Hofmann’s exhaustive methylation of amines.
Answer:
Hofmann’s Exhaustive alkylation : When a primary amine is heated with excess of primary alkyl halide it gives a mixture of secondary amine, tertiary amine along with tetraalkylammonium halide

If excess of alkyl halide is used, tetraalkyl ammonium halide is obtained as major product. The reaction is known as exhaustive alkylation of amines.
Hofmann’s Exhaustive Methylation : The process of converting a primary, secondary or tertiary amine into quaternary ammonium halide by heating them with excess of methyl iodide, is called exhaustive methylation or Hoffmann’s exhaustive methylation.
Thus when methyl amine is heated with excess of methyl iodide it forms dimethylamine (secondary amine), then trimethylamine (a tertiary amine) and finally of quaternary ammonium iodide. The reaction is carried out in the presence of mild base NaHCO3, to neutralize the large quantity of HI formed.

Question 38.
Predict the products of exhaustive methylation of following compounds.
(1) Ethylamine.
Answer:
A primary amine, ethylamine (CH3 – CH2 – NH2) on exhaustive methylation, i.e., on heating with excess methyl iodide, forms secondary amine, tertiary amine and finally a quaternary ammonium salt, ethyl-trimethyl ammonium iodide.

![]()
(2) Benzylamine.
Answer:
Benzylamine C6H5CH2NH2 on exhaustive methylation i.e., on heating with excess methyl iodide forms benzylmethyl amine, benzyldimethyl ammonium chloride and finally benzyltrimethyl ammonium iodide.
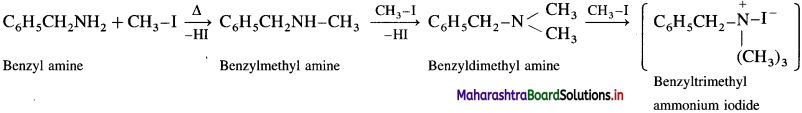
Question 39.
Explain Hofmann elimination.
OR
Write a note on Hoffmann elimination.
Answer:
When tetra alkyl ammonium halide is heated with moist silver hydroxide, a quaternary ammonium hydroxide is obtained. Quaternary ammonium hydroxides are deliquescent crystalline solids and are basic in nature. Quaternary ammonium hydroxides on strong heating undergo ^-elimination to give tertiaryamine, alkenes and water, the reaction is called Hofmann elimination. The major product is least substituted alkene.

Question 40.
Write the bond line formula of the alkene which is obtained as major product from the following amines, on heating with excess of methyl iodide followed by strong heating with moist silver oxide.
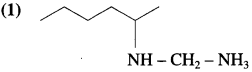
Answer:
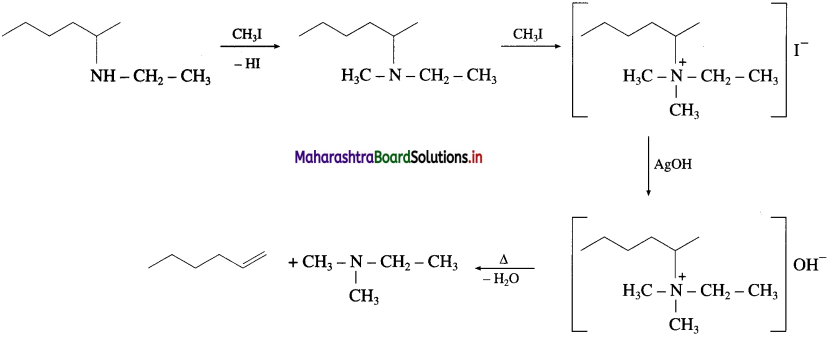
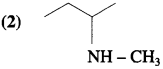
Answer:
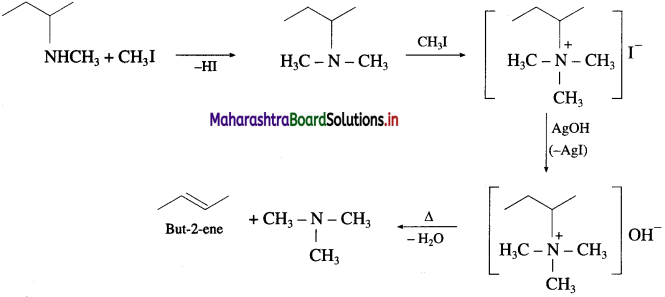
![]()
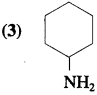
Answer:
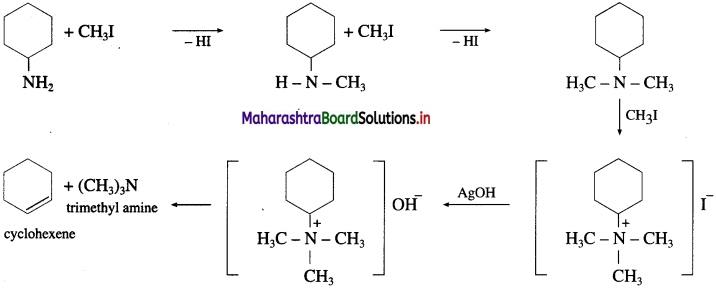
Question 41.
Compound X with a molecular formula C5H13N did not react with nitrous acid, but reacted with one mole of CH3I to form a salt. What is the structure of X?
Answer:
The structure of compound X is 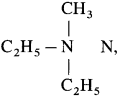 ethyl-N-methylethanamine since compound X is tertiary amine. It reacts with one mole of CH3I to give a quaternary ammonium salt.
ethyl-N-methylethanamine since compound X is tertiary amine. It reacts with one mole of CH3I to give a quaternary ammonium salt.
Question 42.
What is the action of acetyl chloride on :
(1) ethyl amine (ethanamine)
(2) diethyl amine (N-Ethylethanamine)
(3) triethyl amine?
OR
Write a short note on acylation of amines.
Answer:
The reaction of amines with acetyl chloride is called acetylation of amines.
(1) Acetyl chloride on reaction with ethylamine forms monoacetyl derivative, N-ethylacetamide (or N-acetyl ethylamine).

(2) Diethyl amine on reaction with acetyl chloride forms N-acetyl dimethylamine.

(3) Triethyl amine, being a tertiary amine does not have H atom attached to nitrogen of amine, hence it does not react with acetyl chloride.

Question 43.
What is the action of acetic anhydride on aniline?
Answer:
Aniline on reaction with acetic anhydride forms N-phenyl acetamide.

Question 44.
What is the action of benzoyl chloride on ethanamine?
Answer:
When benzoyl chloride is treated with ethanamine, N-ethyl benzamide is obtained.

![]()
Question 45.
What is the action of nitrous acid on ethylamine?
Answer:
Ethyl amine on reaction with nitrous acid in cold forms aliphatic diazonium salt, (unstable intermediate), which decomposes immediately by reaction with solvent water to produce ethyl alcohol and nitrogen gas.

Question 46.
What is the action of nitrous acid on aniline?
Answer:
Aniline reacts with nitrous acid in cold to form diazonium salt which has reasonable solubility at 273 K
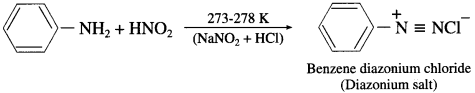
Question 47.
How is benzenediazon|um chloride prepared?
Answer:
Benzenediazonium chloride is prepared by the action of nitrous acid on aniline at 273-278 K. Nitrous acid being unstable, is prepared in situ by the reaction between sodium nitrite and dilute hydrochloric acid.

Question 48.
Write resonance stabilized structures of aryl diazonium salt.
Answer:
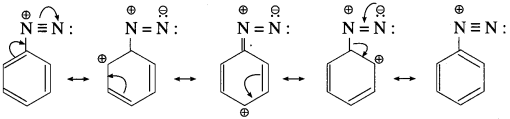
Question 49.
Write a note on Sandmeyer’s reaction.
OR
How is aryl chloride or aryl bromide or aryl cyanide prepared from diazonium salt?
Answer:
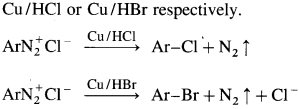
[Replacement by Cl–, Br– and -CN : Sandmeyer reaction.] Freshly prepared aromatic diazonium salt on reaction with cuprous chloride gives aryl chloride, on reaction with cuprous bromide gives aryl bromide and on reaction with cuprous cyanide give aryl cyanide. The reaction in which copper (I) salts are used to replace nitrogen in diazonium salt is called Sandmeyer reaction.

Question 50.
How is aryl chloride or aryl bromide prepared by Gattermann reactions?
Answer:
The aryl chloride or bromides can also be prepared by Gattermann reactions in which diazonium salt reacts with
Question 51.
How is aryl iodide obtained from diazonium salt?
Answer:
When diazonium salt is warmed with potassium iodide, aryl iodide is obtained.
![]()
![]()
Question 52.
Explain the reduction of arene diazonium salt?
OR
How is arene obtained from arene diazonium salt?
OR
What is the action of benzene diazonium chloride on ethanol?
Answer:
Arene diazonium salt on treatment with mild reducing agents like phosphinic acid (hypophosphoric acid) or ethanol, arene is obtained.
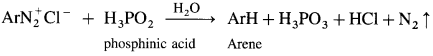
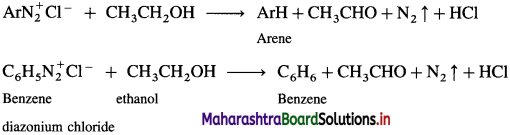
Question 53.
How is phenol obtained from arene diazonium salt?
Answer:
When arene diazonium salt is slowly added to a large volume of boiling dilute sulphuric acid, phenol is obtained,

Question 54.
How is aryl fluoride obtained from diazonium salt?
Answer:
When fluoroboric acid is treated with the solution of diazonium salt, a precipitate of diazonium fluoroborate is obtained, which is filtered and dried. When dry diazonium fluoroborate is heated, it decomposes to give aryl fluoride. This reaction is called Balz-Schiemann reaction.
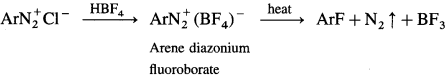
Question 55.
How is nitrobenzene obtained from benzene diazonium fluoroborate?
Answer:
When benzene diazonium fluoroborate is heated with aqueous solution of sodium nitrite in the presence of copper powder, nitrobenzene is obtained.
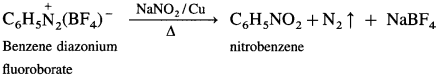
Benzene diazonium fluorobate can be obtained by reaction of benzene diazonium chloride with HBF4.
![]()
Question 56.
What is meant by a coupling reaction? Explain with suitable examples.
OR
What is the action of benzene diazonium chloride on (a) phenol in alkaline medium (b) aniline?
OR
Write a note on the coupling reaction.
Answer:
Diazonium salts react with certain aromatic compounds having an electron-rich group (e.g.-OH, – NH2, etc.) to form azo compounds. This reaction is an electrophilic substitution and is called coupling reaction. Azo compounds are brightly coloured and are used as dyes and indicators. Coupling reaction is an electrophilic substitution reaction. Benzene diazonium chloride reacts with alkaline solution of phenol to give p-hydroxy azo benzene (orange dye).
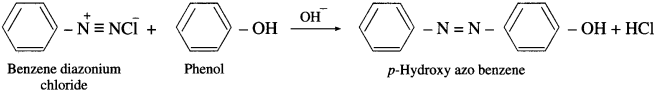
Benzene diazonium chloride reacts with aniline in mild alkaline medium to give p-aminobenzene (yellow dye).
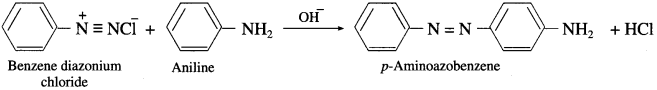
Question 57.
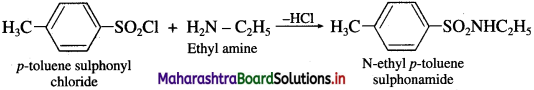
What is the action of p-toluene sulphonyl chloride on ethyl amine and diethyl amine?
Answer:
(1) When ethyl amine is treated with p-toluene sulphonyl chloride, N-ethyl p-toluene sulphonamide is obtained.
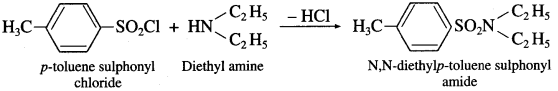
(2) When diethyl amine is treated with p-toluene suiphonyl chloride. N.N-dicthyl p-toluene suiphonyl amide is formed.
![]()
Question 58.
How will you distinguish between :
(1) Ethylamine, diethyl amine and triethyl amine by using (i) nitrous acid (ii) Hinsberg’s reagent.
(2) Diethyl amine and triethyl amine by using acetic anhydride.
Answer:


Question 59.
Give a chemical test to distinguish between following pairs of compounds.
(i) Ethylamine and diethyl amine :
Answer:
Ethylamine (C2H5NH2) is a primary amine while diethyl amine ( (C2H5)2NH) is a secondary amine. So the two can be distinguished by the following test.

![]()
(ii) Ethyl amine and aniline :
Answer:
Ethylamine is an aliphatic amine, while aniline is an aromatic amine. So the two can be distinguished by the following test :

(iii) Aniline and benzyl amine :
Answer:
(iv) Aniline and N-ethylaniline :
Answer:
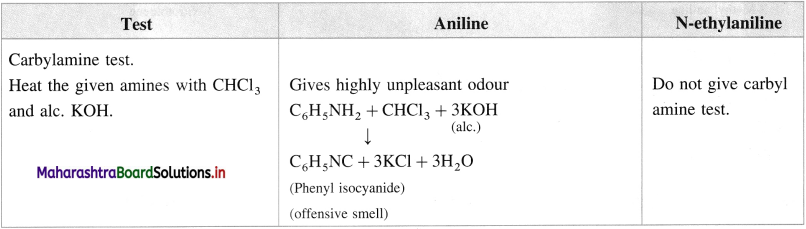
![]()
Question 60.
Compound ‘X’ with a molecular formula C4H11N did not react with Hinsberg’s reagent, but reacted with one mole of CH3I to form a salt. What is the structure of ‘X’?
Answer:
The structure of compound ‘X’ is :

Since the compound ‘X’ does not react with NaN02 and HC1 i.e. nitrous acid (HO – N = O), it must be a tertiary amine.
The tertiary amine reacts with one mole of CH3I to give a quaternary ammonium salt.

Question 74.

p-(dimethylamino) azobenzene is yellow dye which was formerly used as a colouring agent in margarine. Write the structures of the reactants used in the preparation of this dye.
Answer:
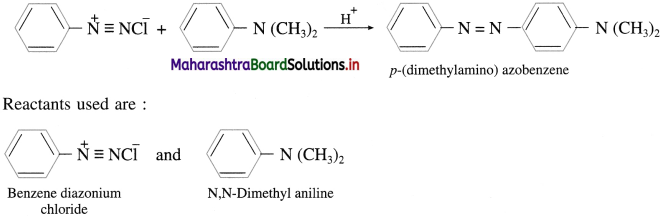
Question 61.
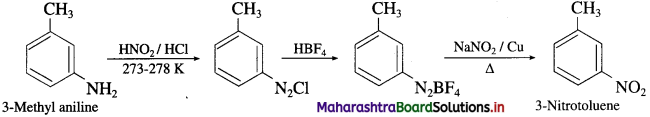
Convert 3-Methyl aniline into 3-nitrotoluene.
Answer:
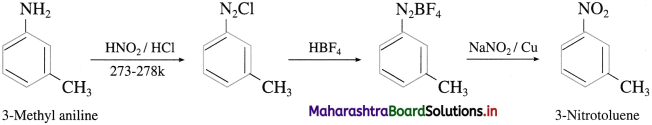
Question 62.
How will you bring about following conversions?
(1) N.Methyl aniline into N-methyl benzanilide.
Answer:

(2) 1.4-Dichlorobutane Into hexane-1,6-diamlne.
Answer:

(3) Benzene into 3-bromo aniline.
Answer:
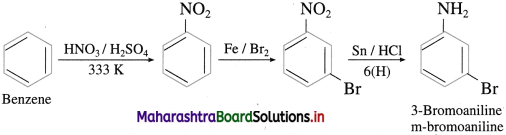
(4) Chlorobenzene into 4-chioroanilinc.
Answer:
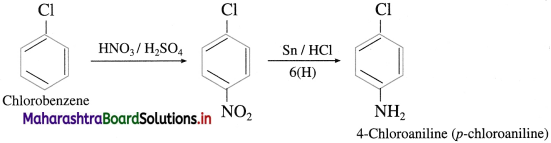
![]()
(5) 11enaniide into toluene.
Answer:

Question 63.
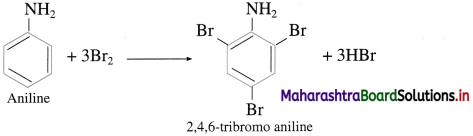
What is the action of aqueous bromine on aniline?
Answer:
Action of aqueous bromine on aniline : When aniline is treated with bromine water at room temperature, a white precipitate of 2, 4, 6-tri bromoaniline is obtained.
Question 64.
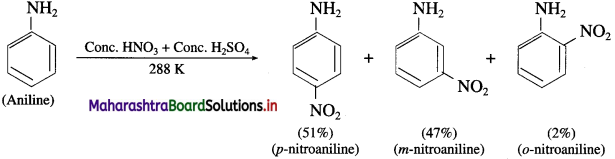
Explain the action of cone, nitric acid (nitrating mixture) on aniline.
Answer:
When aniline is warmed with a mixture of cone, nitric acid and cone, sulphuric acid (a nitrating mixture), a mixture of ortho, meta and para nitroaniline is obtained.
Question 65.
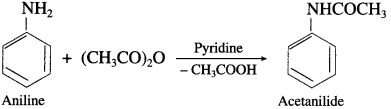
What is the action of acetic anhydride on aniline?
Answer:
When aniline is heated with acetic anhydride, an acetanilide is obtained.
Question 66.
How will you convert aniline to p-nltroanhline? (major product)
Answer:

Question 67.
What is the action of cone, sulphuric acid on aniline?
Answer:
Aniline on treatment with cold sulphuric acid forms anilium hydrogen sulphate which on heating with sulphuric acid at 453 K-475 K gives sulphanilic acid, (p-aminobenzene sulphonic acid) as major product.
Sulphanilic acid exists as a salt; called dipolar ion or zwitter ion. It is produced by the reaction between an acidic group and a basic group present in the same molecule.
![]()
Question 68.
How will you convert the following?
(1) Ethylamine to ethyl alcohol.
Answer:

(2) N-Methyl aniline to N-Nitroso-N-methyl aniline.
Answer:
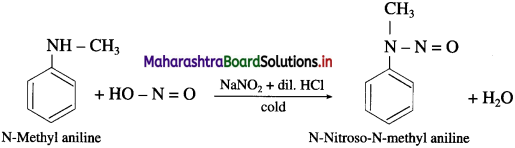
(3) Diethylamine to N-nitrosodiethylamine
Answer:
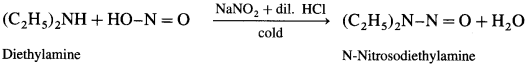
(4) Triethylamine to triethyl ammonium nitrite.
Answer:
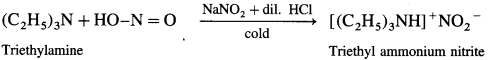
(5) Ethyl amine to N-ethylacetamide.
Answer:

(6) Diethyl amine to N-acetyl diethylamine.
Answer:

(7) Aniline to acetanilide.
Answer:

(8) Aniline to N-ethyl henzamide.
Answer:
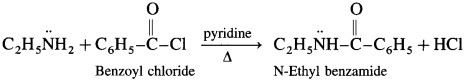
(9) Ethylamine to ethyl isocyanide.
Answer:

(10) Aniline to phenyl isocyanide.
Answer:

(11) Aniline to 2,4,6-tribromoaniline.
Answer:
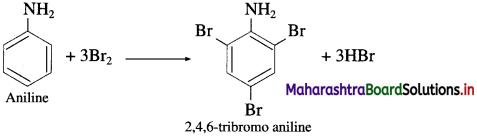
![]()
Question 69.
Give a plausible explanation for each of the following statements :
(1) Ethylamine is soluble in water whereas aniline is not.
Answer:
Ethylamine is soluble in water due to intermolecular hydrogen bonding resulting in the formation of C2H5NH3 ion. Whereas in anline the hydrogen bonding with water is negligible due to the phenyl group (C6H5) is bulky and has -I effect. Therefore, aniline is nearly insoluble in water.

(2) Butan-1-ol is more soluble in water than butani-amine.
Answer:
Rutan- l-al is more soluble in watcr duc to intermoiccular hydrogen bonding. In alcohols, hydrogen bonding is through oxygen atoms. WIereas hutani-amine is less soluble in water due to the larger hydrocarbon part is hydrophobic in nature. Hence, butan-l-ol is more soluble in water than butani-amine.
(3) Butan-1-amlne has higher boiling point than N-ethylethanamine.
Answer:
Due to the presence of two H-atoms on N-atom in butait- I -amine, they undergo extensive intermolecular H-bonding while in N-cthylethanamine due to the presence of one-H atom on the N-atom, they undergo least intermolecular H-bonding. Hence, butan- l-amine has higher boiling point than-N-ethyl ethanamine.
(4) AnIline Is less basic than ethyl afine.
Answer:
Aniline (Kb4-2 x 10-10) is less basic than ethyl amine (Kb5.1 x 10-4). This is because -I effect of phenyl group in aniline as compared to + 1 effect of ethyl group in ethyl amine.

Due to resonance, the lone pair of electrons on the nitrogen atom gets delocalized over the benzene ring and thus less available for protonation. On the other hand, in ethyl anine, delocalization of the lone pair of electrons on the nitrogen atom by resonance is not possible. Further more, the electron density on the nitrogen atom is increased by +1 effect of the ethyl group. Hence, aniline is less basic than ethyl amine.
(5) pKb value of diethyl amine is less than that of ethyl amine.
Answer:
The basic strength of amines is expressed in terms of pKb values. Smaller is the value of pKb more basic is the amine. The pKb value of ethyl amine is 3.29 and that of diethyl amine is 3.00. Therefore, diethyl amine is more basic than ethyl amine.
(6) Aniline cannot be prepared by Gabriel phthalimide synthesis.
Answer:
In Gabriel-phthalimide synthesis of aniline, potassium phthalimide requires the treatment with chlorobenzene or bromobenzene. Since aryl halides do not undergo nucleophilic substitution reaction. Therefore, chlorobenzene or bromobenzene does not react with potassium phthalimide to give N-phenylphthalimide and hence aniline cannot be prepared by Gabriel phthalimide synthesis.
(7) Gabriel phthalimide synthesis is preferred for the preparation of aliphatic primary amines.
Answer:
In aromatic amines, the lone pair of electrons on the N-atom is delocalized over the benzene ring. As a result electron density on the nitrogen atom decreases. Whereas in aliphatic primary amines, due to +1 effect of alkyl group, electron density on nitrogen atom increases. As the pKh value of aliphatic amines is more than that of aromatic amines, aromatic amines are less basic than primary aliphatic amines. Hence, Gabriel phthalimide synthesis is preferred for the preparation of aliphatic amines.
(8) Arere diazonium salts are relatively more stable than alkyl diazonium salts.
Answer:
Arene diazonium salts are stable due to the dispersal of the positive charge over the benzene ring as shown below :

Alkane diazonium salts are unstable due to their tendency to eliminate a stable molecule of nitrogen to form carbocation. Aromatic diazonium salts have much lower tendency to remove nitrogen than aliphatic diazonium salts. Hence, arene diazonium salts are relatively more stable than alkyl diazonium salts.
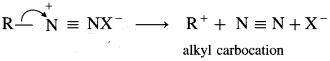
![]()
(9) Tertiary amines cannot be acylated.
Answer:
Tertiary amines do not react with acetic anhydride or acetyl chloride i.e. they can be acylated because they do not contain a H-atom on the N-atom.
(10) Besides the ortho and para derivatives, considerable amount of meta derivatives is also formed during nitration of aniline.
OR
Although amino group is o- and p-directing in electrophilic substitution reactions, aniline on nitration gives substantial amount of m-nitroaniline.
Answer:
In aromatic amines, -NH2 is an electron releasing or activating group. It activates the ortho and para positions in the benzene ring towards electrophilic substitution. When aniline is treated with nitrating mixture (cone. HNO3+ cone. H2SO4), a mixture of ortho and para nitroaniline is obtained. However, a substantial amount of m-nitroaniline is also formed. Aniline being a base gets protonated in acidic medium to form anilium cation, which deactivates the ring and the substitution takes place at the meta position.
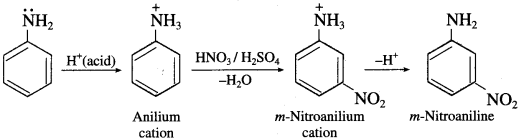
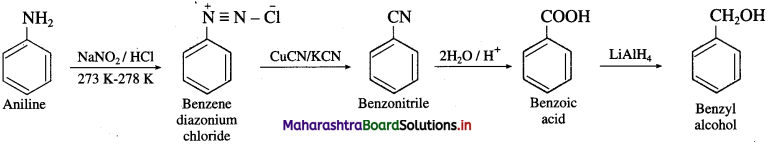
Question 70.
How will you convert :
(1) Aniline into benzyl alcohol.
Answer:
(2) Aniline into 4-bromoaniline.
Answer:
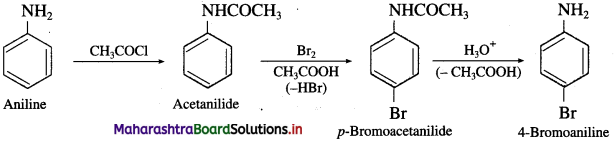
(3) Aniline into 1,3,5-tribromo benzene.
Answer:
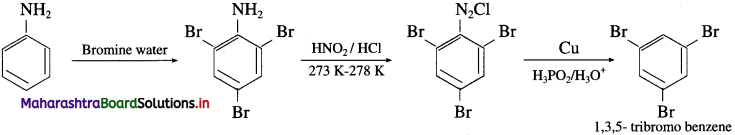
(4) Aniline into 2,4,6-tribromo fluoro benzene.
Answer:

Question 71.
How will you convert :
(1) Propanoic acid into ethanoic acid.
Answer:
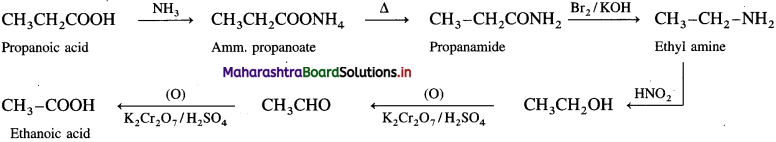
(2) Propanoic acid into ethanol
Answer:

(3) Ethanamine into propan-l-amine.
Answer:

![]()
(4) Propan-l-amine into ethanamine.
Answer:
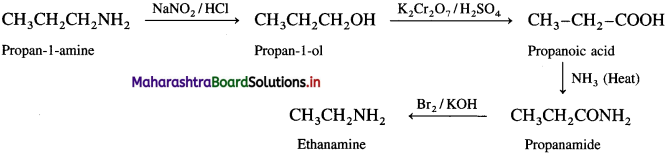
(5) Propanoic acid into ethanamine.
Answer:

(6) Ethanamine into propanoic acid.
Answer:
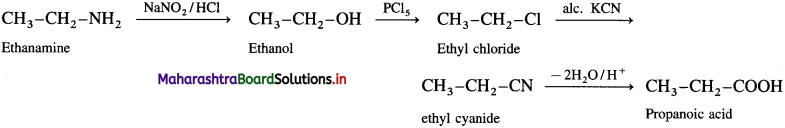
(7) Benzene to aniline.
Answer:
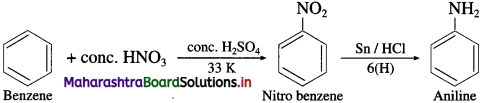
(8) Aniline to Benzene.
Answer:
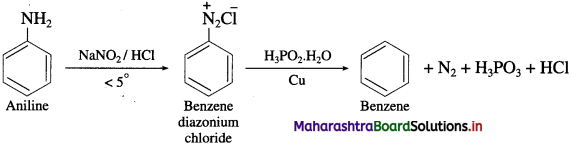
(9) Aniline into benzoic acid.
Answer:
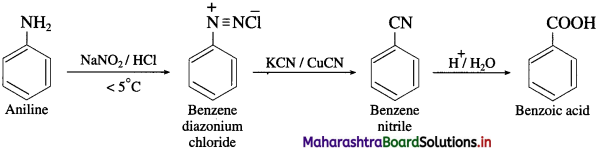
(10) Benzoic acid into aniline.
Answer:

(11) Aniline into benzamide.
Answer:
![]()
(12) 3-Nitrotoluene into 3-methyl aniline.
Answer:

(13) 3-Methyl aniline into 3-Nitrotoluene.
Answer:
Question 72.
An organic compound ‘A’ having molecular formula C2H6O evolves hydrogen gas on treatment with sodium metal and on treatment with red phosphorous and iodine gives compound ‘B’. The compound ‘B’ on treatment with alcoholic KCN and on subsequent reduction gives compound ‘C’. The compound ‘C’ on treatment with nitrous acid evolves nitrogen gas. Write the balanced chemical equations for all the reactions involved and identify the compounds ‘A’, ‘B’ and ‘C;.
Answer:
A = C2H5OH ethanol
B = C2H5I ethyl iodide
C = C2H5CH2NH2 n-propyl amine
Compound C2H6O = C2H5OH

Question 73
Identify B, C and D write complete reactions :
![]()
Answer:

![]()
Question 74.
Identify the compounds B, C and D in the following series of reactions and rewrite the complete equations :
![]()
Answer:

Question 75.
Identify the compounds ‘A’ and ‘B’ in the following equation :
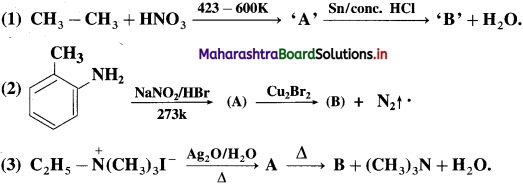
Answer:

Question 76.
Answer in one sentence :
(1) Arrange the following compounds in decreasing order of basic strength in their aqueous solutions. NH3, C2H5NH2, (CH3)2NH, (CH3)3N
Answer:
The decreasing order of basic strength is – (C2H5)2NH > (C2H5)3N > (C2H5)2NH > NH3
(The reason that ethyl group has greater +1 effect than methyl group).
(2) Arrange the following compounds in an increasing order of their solubility in water.
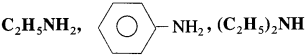
Answer:
The solubility increases in order in which molecular mass decreases.

![]()
(3) What is Hinsberg’s reagent?
Answer:
Benzenesulphonyl chloride (C6H5SO2Cl) is known as Hinsberg’s reagent.
(4) Name the reaction in which a primary amine is formed from amide.
Answer:
Hoffmann bromamide degradation.
(5) NH3 is a Lewis base.
Answer:
Since nitrogen in ammonia molecule has a lone pair of electrons, it is a Lewis base.
(6) How many primary amines are possible for the compound C3H9N?
Answer:
For the compound C3H9N, two primary amines are possible.
(7) State the hybridization of the nitrogen atom in amines.
Answer:
The hybridization of nitrogen atom in amines is sp3.
(8) Arrange the following compounds in an increasing order of basic strength. Aniline, p-nitroaniline, p-toluidine.
Answer:
p-nitroaniline < aniline < p-toluidine.
(9) Which of the two is more basic and why? CH3NH2 or NH3
Answer:
Due to +1 effect of -CH3 group, electron density on N-atom increases, hence methyl amine is a stronger base than ammonia.
(10) Which of the two is more basic and why? p-toluidine or aniline.
Answer:
p-toluidine is more basic due to the presence of -CH3 group at para position. Due to +1 effect of -CH3 group, electron density on nitrogen increases, hence the tendency to donate pair of electrons increases.
Multiple Choice Questions
Question 77.
Select and write the most appropriate answer from the given alternatives for each subquestion :
1. Which of the following is an amine?
(a) C2H5N(COCH3)2
(b) (C2H5)2N – N = 0
(c) (C2H5)3N
(d) All of these
Answer:
(d) All of these
2. N-methyl-N-ethyl-n-propyl amine is
(a) a primary amine
(b) a secondary amine
(c) a tertiary amine
(d) an alkyl nitrile
Answer:
(c) a tertiary amine
3. Which of the following is a tertiary amine?

Answer:
(d)
4. Tertiary butyl amine is a
(a) primary amine
(b) secondary amine
(c) tertiary amine
(d) quaternary ammonium salt
Answer:
(a) primary amine
5. The IUPAC name of

(a) ethyl propanamine
(b) ethyl butylamine
(c) 2-pentanamine
(d) 3-hexanamine
Answer:
(d) 3-hexanamine
![]()
6. The IUPAC name of ethyl dimethyl amine is ……………..
(a) 2-amino propane
(b) N,N-dimethyl ethanolamine
(c) ethyl methanamine
(d) propanamine
Answer:
(b) N,N-dimethyl ethanolamine
7. Isopropyl amine and trimethyl amine are ……………..
(a) acidic in nature
(b) electrophilic compounds
(c) structural isomers
(d) optically active compounds
Answer:
(c) structural isomers
8. N, N-dimethylethanolamine is ……………

Answer:
(b)
9. IUPAC name of diethylmethyl amine is ………………
(a) methyl amino propane
(b) N-Ethyl-N-methylhexanamine
(c) methyl diethanamine
(d) amino pentane
Answer:
(b) N-Ethyl-N-methylhexanamine
10. Ethyl bromide reacts with excess of alcoholic ammonia, the major product is …………..
(a) ethyl amine
(b) diethylamine
(c) triethylamine
(d) tetraethyl ammonium bromide
Answer:
(a) ethyl amine
11. Isopropylamine is obtained by the reduction of
(a) acetoxime
(b) acetaldoxime
(c) formaldoxime
(d) aldoxime
Answer:
(a) acetoxime
12. Which of the following compounds can be converted into amines in the presence of Na and alcohol?
(a) Alkyl nitriles
(b) Aldoxime
(c) Ketoxime
(d) All of these
Answer:
(d) All of these
13. Chloroethane when boiled with excess of aqueous-alcoholic ammonia gives hydrochloric acid and
(a) triethyl amine
(b) trimethyl amine
(c) diethyl amine
(d) ethyl amine
Answer:
(d) ethyl amine
14. How many hydrogen atoms are required for the reduction of 1-nitropropane to n-propyl amine?
(a) Four
(b) Three
(c) Six
(d) Two
Answer:
(c) Six
![]()
15. A secondary alkyl halide is heated with excess of ammonia, the major product obtained is
(a) primary amine
(b) secondary amine
(c) tertiary amine
(d) quaternary ammonium salt
Answer:
(a) primary amine
16. The true statement about ethylamine is
(a) it is weaker base than ammonia
(b) it is stronger base than diethyl amine
(c) it is stronger base than triethyl amine
(d) it is stronger base than alkali
Answer:
(c) it is stronger base than triethyl amine
17. The reaction which is given only by primary amines is
(a) acetylation
(b) alkylation
(c) reaction with HNO2
(d) carbyl amine test
Answer:
(d) carbyl amine test
18. The amine which reacts with NaNO2 and dil. HCl to give yellow oily compound is
(a) ethylamine
(b) isopropylamine
(c) sec-butylamine
(d) dimethylamine
Answer:
(d) dimethylamine
19. The name of the compound ‘C’ in the following series of reactions, is ![]()
(a) propan-l-ol
(b) propan-2-ol
(c) butan-l-ol
(d) butan-2-ol
Answer:
(b) propan-2-ol
20. Triethylamine when treated with nitrous acid gives
(a) an alcohol
(b) a nitrosamine
(c) a monoacetyl derivative
(d) a soluble nitrite salt
Answer:
(d) a soluble nitrite salt
21. Ammes are basic in nature because
(a) of the nitrogen atom contain or lone pair of electrons
(b) they give H+ ions in aqueous medium
(c) they form quaternary ammonium salts when heated with acids
(d) both (a) and (c)
Answer:
(a) of the nitrogen atom contain or lone pair of electrons
22. An aqueous solution of primary amine contains
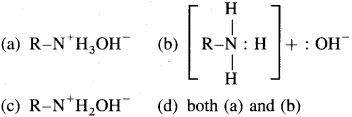
Answer:
(d)
23. The basic nature of amines in an aqueous solution is in the order of
(a) tert. > sec. > pri.
(b) sec. > pri. > tert.
(b) pri. > sec. > tert.
(d) pri. > tert. > sec.
Answer:
(b) pri. > sec. > tert.
24. In trimethyl ammonium ion, the number of sigma bonds attached to nitrogen are
(a) 2
(b) 3
(c) 4
(d) 5
Answer:
(b) 3
![]()
25. The number of coordinate bond/bonds in a trialkyl ammonium ion is
(a) one
(b) two
(c) three
(d) four
Answer:
(a) one
26. The number of electrons in the valence shell of nitrogen in methyl amine is
(a) 5
(b) 3
(c) 8
(d) 7
Answer:
(c) 8
27. Ethanamine reacts with excess of acetyl chloride to form
(a) C2H5NHCOCH3
(b) C2H5N(CH3)2
(c) C2H5N(COCH3)2
(d) C2H5N+H3Cl
Answer:
(c) C2H5N(COCH3)2
28. The compound used for acylation of amine is
(a) (CH3CO)2O
(b) CH3COOH
(c) CH3COCl
(d) both (a) and (c)
Answer:
(d) both (a) and (c)
29. Dimethyl amine reacts with acetyl chloride to give
(a) N-acetyl methyl amine
(b) N-acetyl ethyl amine
(c) N-acetyl dimethyl amine
(d) N-acetyl diethyl amine
Answer:
(c) N-acetyl dimethyl amine
30. Identify ‘A’ in the following reaction :

Answer:
(c)
31. n-propyl alcohol is obtained when HNO2 is treated with
Answer:
(c)
32. A mixture of CH3NH2, (CH3)2NH, (CH3)3N can be distinguished by using
(a) HCI
(b) HNO2
(c) HNO3
(d) H2SO4
Answer:
(b) HNO2
33. In the acetylation reaction the H-atom of an amine is replaced by
(a) a carbonyl group
(b) an alkyl group
(c) an acetyl group
(d) an imino group
Answer:
(c) an acetyl group
![]()
34. Amines are basic in nature
(a) as they have a fishy odour
(b) as they form quaternary ammonium salts with alkyl halides
(c) due to the presence of an unshared pair of electrons on the nitrogen atom
(d) all of these
Answer:
(c) due to the presence of an unshared pair of electrons on the nitrogen atom
35. The correct order of increasing basic strength is
(a) NH3 < CH3NH2 < (CH3)2NH
(b) CH3NH2 < (CH3)2NH < NH3
(c) CH3NH2 < NH3 < (CH3)2NH
(d) (CH3)2NH < NH3 < CH3NH2
Answer:
(a) NH3 < CH3NH2 < (CH3)2NH
36. Which of the following is the strongest base?

Answer:
(d)
37. Identify the weakest base amongst the following :
(a) p-methoxyaniline
(b) o-toluidine
(c) benzene-1, 4-diamine
(d) 4-aminobenzoic acid
Answer:
(d) 4-aminobenzoic acid
38. Amine that cannot be prepared by Gabriel phthalimide synthesis is
(a) aniline
(b) benzyl amine
(c) methyl amine
(d) iso-butyl amine
Answer:
(a) aniline
39. Which of the following exist as Zwitter ion?
(a) Salicylic acid
(b) Suphanilic acid
(c) p-Aminophenol
(d) p-Amino acetophenone
Answer:
(b) Suphanilic acid
40. Reduction of benzene diazonium chloride with Zn/HCl gives
(a) phenyl hydrazine
(b) hydrazine hydrate
(c) aniline
(d) ozo benzene
Answer:
(c) aniline
41. When primary amine reacts with CHCl3 in alcoholic KOH, the product is
(a) aldehyde
(b) alcohol
(c) cyanide
(d) an isocyanide
Answer:
(d) an isocyanide
42. Which of the following amines cannot be prepared by Gabriel phthalimide synthesis?
(a) sec-Propylamine
(b) tert-Butylamine
(c) 2-Phenylethylamine
(d) N-Methyl benzyl amine
Answer:
(d) N-Methyl benzyl amine
![]()
43. Which of the following compounds has highest boiling point?
(a) Ethane
(b) Ethanoic acid
(c) Ethanol
(d) Ethanamine
Answer:
(b) Ethanoic acid
44. Identify the statement about the basic nature of amines.
(a) Alkylamines are weaker bases than ammonia.
(b) Arylamines are stronger bases than alkylamines.
(c) Secondary aliphatic amines are stronger bases than primary aliphatic amines.
(d) Tertiary aliphatic amines are weaker bases than arylamines.
Answer:
(c) Secondary aliphatic amines are stronger bases than primary aliphatic amines.
45. The compounds ‘A’, ‘B’ and ‘C’ react with methyl iodide to give finally quaternary ammonium iodides. Only ‘C’ gives carbylamines test while only ‘A’ forms yellow oily compound on reaction with nitrous acid. The compounds ‘A’, ‘B’ and ‘C’ are respectively.
(a) butan-1-amine, N-ethylethanamine and
N, N-dimethylethanamine.
(b) N-ethylethanamine, N, N-dimethylethanamine and butan-1 – amine.
(c) N, N-dimethylethanamine, N-ethylethanamine and butan-1-amine.
(d) N-ethylethanamine, butan-1-amine and N-ethylethanamine.
Answer:
(b) N-ethylethanamine, N, N-dimethylethanamine and butan-1 – amine.
46. Which of the following amines is most basic in nature?
(a) 2, 4-Dichloroaniline
(b) 2, 4-Dimethylaniline
(c) 2, 4-Dinitroaniline
(d) 2, 4-Dibromoaniline
Answer:
(b) 2, 4-Dimethylaniline
47. How many moles of methyl iodide are required to convert ethylamine, diethylamine and triethylamine into quaternary ammonium salt, respectively?
(a) 1, 2 and 3
(b) 2, 3 and 1
(c) 3, 2 and 1
(d) 3, 1 and 2
Answer:
(c) 3, 2 and 1
48. Which of the following amines does not undergo acetylation?
(a) t-Butylamine
(b) Ethylamine
(c) Diethylamine
(d) Triethylamine
Answer:
(d) Triethylamine
49. n-Propylamine can be prepared by catalytic reduction of
(a) n-propyl cyanide
(b) propionaldoxime
(c) acetoxime
(d) nitroethane
Answer:
(b) propionaldoxime
50. Identify the compound ‘B’ in the following series of reactions :
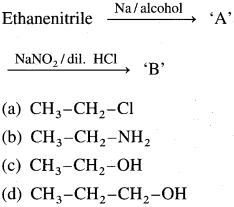
Answer:
(c)
![]()
51. Chloropicrin is used as
(a) antiseptic
(b) antibiotic
(c) insecticide
(d) anaesthetic
Answer:
(c) insecticide
52. Identify the compound B in the following series of reactions. ![]()
(a) n-propyl chloride
(b) propanamine
(c) n-propyl alcohol
(d) Isopropyl alcohol
Answer:
(c) n-propyl alcohol
53. Which of the following amines yields foul smelling product with haloform and alcoholic KOH?
(a) Ethyl amine
(b) Diethyl amine
(c) Triethyl amine
(d) Ethyl methyl amine
Answer:
(a) Ethyl amine
54. Which of the following compounds is NOT prepared by the action of alcoholic NH3 on alkyl halide?
(a) CH3NH2
(b) CH3-CH2-NH2
(c) CH3 – CH2 – CH2 – NH2
(d) (CH3)3CNH2
Answer:
(d) (CH3)3CNH2