Balbharti Maharashtra State Board 11th Chemistry Important Questions Chapter 11 Adsorption and Colloids Important Questions and Answers.
Maharashtra State Board 11th Chemistry Important Questions Chapter 11 Adsorption and Colloids
Question 1.
Explain the phenomenon of adsorption with the help of examples.
Answer:
Consider the following two examples:
- Example 1: When a metal spoon is dipped in milk and taken out, it is observed that a film of milk particles covers the spoon’s surface.
- Example 2: If a cold water bottle is taken out from the refrigerator and kept on a table for a while, water vapour is seen to condense on the outer surface of the bottle, forming droplets or a film.
- In the above examples, the milk particles or the water molecules from the air get adsorbed on the surface of the spoon and the bottle, respectively.
- Similarly, the surfaces of many objects around us are exposed to the atmosphere. Water molecules as well as other gas molecules such as N2, O2, from the air form an invisible multimolecular film on these objects. This is known as the phenomenon of adsorption.
Question 2.
Why does adsorption occur?
Answer:
- The adsorption phenomenon is caused by dispersion forces (also known as London dispersion forces or van der Waals forces) which are short-range and additive. Adsorption force is the sum of all interactions between all the atoms.
- The pulling interactions cause the surface of a liquid to tighten like an elastic film.
- A measure of the elastic force at the surface of a liquid is called surface tension.
- There is a tendency to have minimum surface tension, i.e., decrease of free energy, which leads to adsorption.
Question 3.
Define surface tension.
Answer:
A measure of the elastic force at the surface of a liquid is called surface tension.
OR
Surface tension is the amount of energy required to stretch or increase the surface of a liquid by a unit area.
Question 4.
Define the following terms.
i. Adsorbent
ii. Adsorbate
Answer:
i. Adsorbent: The material or substance present in the bulk, on the surface of which adsorption takes place is called adsorbent.
ii. Adsorbate: The substance getting adsorbed on the adsorbent is called as adsorbate.
![]()
Question 5.
Give some examples of adsorption.
Answer:
Following are some examples of adsorption:
- Adsorption of gases like hydrogen and oxygen by finely divided metals, namely, platinum, palladium, copper, nickel, etc.
- Adsorption of gases like nitrogen and carbon dioxide by activated charcoal.
- Removal of colouring matter like an organic dye, for example, methylene blue. When charcoal is added to methylene blue solution and shaken, it becomes colourless after some time as dye molecules accumulate on the surface of charcoal.
Question 6.
What is desorption?
Answer:
The process of removal of an adsorbed substance from a surface on which it was adsorbed is called desorption.
Question 7.
Define sorption.
Answer:
When both adsorption and absorption occur simultaneously, it is known as sorption.
e.g. When a chalk is dipped in ink, the ink molecules are adsorbed at the surface of the chalk while the solvent of the ink goes deeper into the chalk due to absorption.
Question 8.
What is physisorption? State its characteristics.
Answer:
When the adsorbent such as gas molecules are accumulated at the surface of a solid on account of weak van der Waals forces, the adsorption is termed as physical adsorption or physisorption.
Characteristics:
- The van der Waals forces involved in physical adsorption are similar to forces causing condensation of gas into liquid. Thus, heat is released in physisorption.
- The heat released during physisorption is of the same order of magnitude as heat of condensation.
- Due to weak nature of van der Waals forces, physisorption is weak in nature.
- The adsorbed gas forms several layers of molecules at high pressures.
- The extent of adsorption is large at low temperatures.
- The equilibrium is attained rapidly.
- Physisorption is readily reversed by lowering of pressure of gas or by raising temperature.
![]()
Question 9.
Define chemisorption, Write its main features.
Answer:
When the gas molecules accumulate at the surface of a solid or adsorbate by means of chemical bonds (covalent or ionic), the adsorption is termed as chemical adsorption or chemisorption.
Features of chemical adsorption:
- Chemisorption is specific in nature.
- Chemisorption involving the gas-solid as the adsorbate and adsorbent is usually exothermic i.e., heat is released during this process (Exception: The adsorption of hydrogen on glass is endothermic).
- The heat evolved in chemisorption per mole of adsorbate is nearly the same order of magnitude as that accompanying chemical bonding.
- Chemisorption involves a large energy of activation and hence, it is also referred as activated adsorption.
- Chemisorption increases with increase in temperature in the beginning, as a greater number of molecules can have activation energy. But after certain temperature chemisorption decreases with increase in temperature as the chemical bonds break.
- Sometimes at low’ temperature, physisorption occurs which passes into chemisorption as the temperature is raised.
- Chemisorption is dependent on surface area of the adsorbent.
[Note: Chemisorption was first investigated in 1916 by American Chemist, Irving Langmuir (1881-1957).]
Question 10.
Why is chemisorption also known as activated adsorption?
Answer:
Chemisorption involves a large energy of activation and hence, it is also referred as activated adsorption.
Question 11.
Give reason: Adsorption of hydrogen on glass is an endothermic process.
Answer:
Adsorption of hydrogen on glass is an endothermic process because heat is absorbed during the process due to dissociation of hydrogen.
Question 12.
Explain graphically the effect of the following factors on the adsorption of gases by solids.
i. Temperature of the adsorbent surface
ii. Pressure of the gas (adsorbate)
Answer:
i. Temperature of the adsorbent surface:
- Adsorption is an exothermic process.
- According to Te Chatelier’s principle, it is favoured at low temperature.
- Therefore, the amount of gas adsorbed is inversely proportional to the temperature.
- The graph given below shows plots of volume of N? adsorbed per unit mass of adsorbent against the pressure of a gas at different temperatures.
- As temperature increases from 193 K to 273 K at a constant pressure ‘P’, the amount of gas adsorbed decreases.
ii. Pressure of the gas:
- At any temperature, the extent of gas adsorbed increases with an increase in pressure.
- The extent of adsorption is directly proportional to pressure of the gas.
- At high pressures extent of adsorption becomes independent of the pressure. The surface of adsorbent is then almost fully covered by adsorbed gaseous molecules.
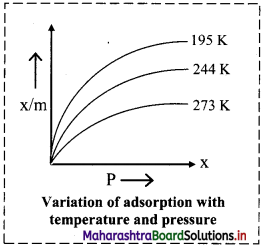
Question 13.
What are the applications of adsorption?
Answer:
Following are the various applications of adsorption:
i. Catalysis (Heterogeneous catalysis):
- The solid catalysts are used in many industrial manufacturing processes.
- For example, iron is used as a catalyst in manufacturing of ammonia, platinum in manufacturing of sulphuric acid, H2SO4 (by contact process) while finely divided nickel is employed as a catalyst in hydrogenation of oils.
ii. Gas masks:
- It is a device which consists of activated charcoal or mixture of adsorbents.
- It is used for breathing in coal mines to avoid inhaling of the poisonous gases.
iii. Control of humidity: Silica and alumina gels are good adsorbents of moisture.
iv. Production of high vacuum:
- Lowering of temperature at a given pressure, increases the rate of adsorption of gases on charcoal powder. By using this principle, high vacuum can be attained by adsorption.
- A vessel evacuated by vacuum pump is connected to another vessel containing coconut charcoal cooled by liquid air. The charcoal adsorbs the remaining traces of air or moisture to create a high vacuum.
v. Adsorption indicators: The adsorption is used to detect the end point of precipitation titrations. Dyes such as eosin, fluorescein are used as indicators.
e.g.
a. A solution of sodium chloride containing a small amount of fluorescein is titrated against silver nitrate solution.
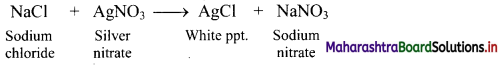
b. When chloride ions are over, fluorescein is adsorbed on white silver chloride precipitate and hence, red colour is developed.
c. Thus, colour changes from pale yellow to reddish pink at the end point.
vi. Separation of inert gases:
- In a mixture of noble gases, different gases adsorb to different extent.
- Due to selective adsorption principle, gases can be separated on coconut charcoal.
vii. Froth floatation process:
- A low-grade sulphide ore is concentrated by separating it from silica and other earthy matter using pine oil as frothing agent.
- Hydrophobic pine oil preferentially adsorbs sulphide ore which is taken up in the froth.
viii. Chromatographic analysis:
- It is based on selective adsorption of ions from solution using powdered adsorbents such as silica or alumina gel.
- It has several industrial and analytical applications. Other applications include surface area determination, purification of water, etc.
![]()
Question 14.
Explain how high vacuum can be obtained by adsorption.
Answer:
- Lowering of temperature at a given pressure, increases the rate of adsorption of gases on charcoal powder. By using this principle, high vacuum can be attained by adsorption.
- A vessel evacuated by vacuum pump is connected to another vessel containing coconut charcoal cooled by liquid air. The charcoal adsorbs the remaining traces of air or moisture to create a high vacuum.
Question 15.
State whether TRUE or FALSE. Correct if false.
i. The rate of adsorption of gases on charcoal powder decreases on lowering of temperature at a given pressure.
ii. Noble gases can be separated from their mixture using the principle of selective adsorption as they adsorb to different extent.
iii. Pine oil is used as frothing agent in froth floatation process.
Answer:
i. False
The rate of adsorption of gases on charcoal powder increases on lowering of temperature at a given pressure.
ii. True
iii. True
Question 16.
Match the following.
| Column A | Column B | ||
| i. | Iron | a. | Hydrogenation of oils |
| ii. | Nickel | b. | Production of sulphuric acid |
| iii. | Platinum | c. | Synthesis of ammonia |
Answer:
i – c,
ii – a,
iii – b
Question 17.
What is a catalyst?
Answer:
A catalyst is a substance which when added to a reacting system, increases the rate of a reaction without itself undergoing any permanent chemical change.
Question 18.
Explain the importance of catalysts in chemical industries.
Answer:
- A large number of the chemicals manufactured in industries make use of catalysts to obtain specific products.
- The use of catalyst lowers the reaction temperature as well as energy costs significantly.
Due to these advantages, catalysts are of great importance in chemical industry.
![]()
Question 19.
Name two types of catalysis.
Answer:
- Homogeneous catalysis
- Heterogeneous catalysis
Question 20.
Define homogeneous catalysis and give any two examples.
Answer:
When the reactants and the catalyst are in the same phase, it is said to be homogeneous catalysis.
e.g.
i. Iodide ion (I–) is used as homogeneous catalyst in decomposition of aqueous hydrogen peroxide because both I– and H2O2 are present in the same aqueous phase.
ii. Hydrolysis of sugar is catalysed by H+ ions furnished by sulphuric acid.
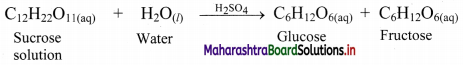
All reactants and catalyst are in same solution phase.
[Note: Enzyme catalysis is also an important type of homogeneous catalysis.]
Question 21.
Justify: Lead chamber process is an example of homogeneous catalysis.
Answer:
i. In the lead chamber process, sulphur dioxide is oxidized to sulphur trioxide with dioxygen (O2) in the presence of nitric oxide as catalyst.
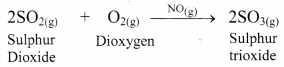
ii. Since all the reactants as well as the catalyst is present in gaseous state. i.e., in same phase, it is a homogeneous catalysis reaction.
Hence, lead chamber process is an example of homogeneous catalysis.
Question 22.
Describe heterogeneous catalysis with the help of one example.
Answer:
i. When the reactants and catalyst are in different phase, it is said to be heterogeneous catalysis.
ii. The heterogeneous catalyst is generally a solid and the reactants may either be gases or liquids.
iii. When the solid catalyst is added to the reaction mixture, it does not dissolve in the reacting system and the reaction occurs on the surface of the solid catalyst.
e.g. Dinitrogen (N2) and dihydrogen (H2) combine to form ammonia in Haber process in the presence of finely divided iron along with K2O and Al2O3.
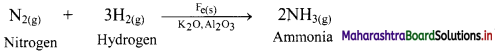
b. In the above reaction, Al2O3 and K2O are promoters of the Fe catalyst. Al2O3 is added to prevent the fusion of Fe particles. K2O causes chemisorption of nitrogen atoms. Molybdenum is also used as promoter.
c. Since the reactants are present in gaseous phase while the catalyst used is in solid phase, it represents heterogeneous catalysis.
Question 23.
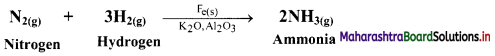
i. State whether the given reaction is an example of heterogeneous or homogeneous catalysis.
ii. What is the role of Fe, K2O and Al2O3 in this reaction?
Answer:
i. This reaction is an example of heterogeneous catalysis.
ii. Fe is used as a catalyst while K2O and Al2O3 are promoters of the Fe catalyst. Al2O3 is used to prevent the fusion of Fe particles while K2O causes chemisorption of nitrogen atoms.
![]()
Question 24.
Describe hydrogenation reaction of vegetable oils.
Answer:
i. Hydrogenation reaction of vegetable oils used in food industry to produce solid fats. The reaction is as follows:
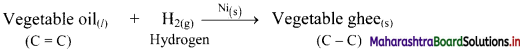
ii. The reaction is catalysed by finely divided metals like Ni, Pd or Pt.
iii. Vegetable oil contains one or more carbon-carbon double bonds (C = C) in its structure.
iv. On hydrogenation, a solid product (which contains only carbon-carbon single bonds) is formed. It is called Vanaspati ghee.
v. The hydrogenation reaction of vegetable oils is an example of heterogeneous catalysis as the reactant and the catalyst are not present in the same phase.
Question 25.
i. Explain the role of catalytic converters in automobile exhaust.
ii. Why do automobiles with catalytic converter require unleaded petrol?
Answer:
i. a. An important application of heterogeneous catalysts is in automobile catalytic converters.
b. In automobile exhaust, large number of air pollutants such as carbon monoxide, nitric oxide, etc. are present.
c. The catalytic converter transforms these air pollutants into carbon dioxide, water, nitrogen and oxygen.
ii. The catalyst used in the catalytic converter gets poisoned by the adsorption of lead (Pb) present in the petrol. Hence, the automobiles with catalytic converter requires unleaded petrol.
Question 26.
What are inhibitors? Explain with an example.
Answer:
Inhibitors are substances that decreases the rate of chemical reactions.
e.g. Chloroform forms poisonous substance, carbonyl chloride, by air oxidation.
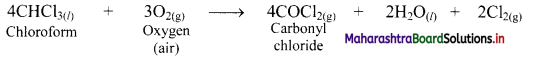
When 2% ethanol is added to chloroform, the formation of COCl2 is suppressed because ethanol acts as an inhibitor and retards the above reaction.
[Note: Chloroform was earlier used as an anaesthetic.]
Question 27.
Write decomposition reaction of hydrogen peroxide. Suggest how this decomposition can be prevented.
Answer:
i. Hydrogen peroxide decomposes as,
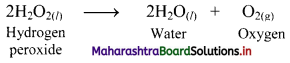
ii. The reaction can be inhibited by addition of dilute acid or glycerol as they act as inhibitors.
Question 28.
Explain why 2% ethanol is added to chloroform?
Answer:
Inhibitors are substances that decreases the rate of chemical reactions.
e.g. Chloroform forms poisonous substance, carbonyl chloride, by air oxidation.
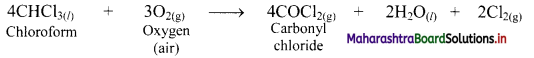
When 2% ethanol is added to chloroform, the formation of COCl2 is suppressed because ethanol acts as an inhibitor and retards the above reaction.
[Note: Chloroform was earlier used as an anaesthetic.]
![]()
Question 29.
Describe the steps involved in heterogeneous catalysis by solid catalyst.
OR
Explain the mechanism involved in catalytic action of a heterogeneous catalyst.
Answer:
The catalytic action of a heterogeneous catalyst occurs on the surface of a catalyst.
The mechanism involves the following five steps.
i. Diffusion of reactants towards the surface of the catalyst.
ii. Adsorption of reactant molecules on the surface of the catalyst.
iii. Occurrence of chemical reaction on the catalyst surface and formation of an intermediate.
iv. Formation of the products.
v. Desorption of reaction products from the catalyst surface. Products leave the catalyst surface in the following steps.
Steps involved in desorption of reaction products:
Diffusion → Adsorption → Intermediate formation → Product formation → Desorption
vi. Fresh reactant molecules can replace the products to start the cycle again as in first step.
vii. This is why catalyst remains unchanged in mass and chemical composition at the end of the reaction.
Question 30.
Write a short note on catalytic activity.
Answer:
- The catalytic activity of a catalyst depends on the strength of chemisorption.
- If large number of reactant molecules (gas or liquid) are strongly adsorbed on the surface of solid catalyst, the catalyst is said to be active.
- However, the adsorption of reactant molecules on the surface, that is, the bond formed between adsorbate and adsorbent surface should not be very strong so that they are not immobilized.
- d-block metals such as Fe, V and Cr tend to be strongly active towards O2, C2H2, C2H4, CO, H2, CO2, N2, etc.
- Mn and Cu are unable to adsorb N2 and CO2.
- The metals Mg and Li adsorb O2 selectively.
Question 31.
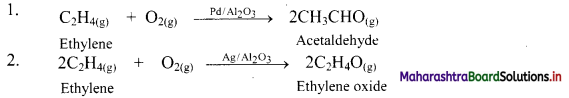
Explain catalytic selectivity with suitable examples.
Answer:
i. Some solid catalysts are selective in their action.
ii. The same gaseous reactants produce different products when different catalysts are used.
e.g.
a. The gaseous ethylene and O2 react to produce different products with different catalysts.
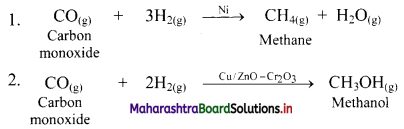
b. The gaseous carbon monoxide and H2 produce different products by using different catalysts.
Question 32.
i. What are zeolites?
ii. Zeolites are shape selective catalysts. Explain.
iii. What is the use of a zeolite catalyst ZSM-5 in petroleum industry?
Answer:
i. a. Zeolites are aluminosilicates with three-dimensional network of silicates.
b. Some silicon atoms in this network are replaced by aluminium atoms giving Al – O – Si framework which results in microporous structure.
ii. a. The reactions in zeolites are dependent on the size and shape of reactant or products, b. It also depends on the pores and cavities of zeolites.
b. Therefore, zeolites are shape selective catalysts.
iii. In petroleum industry, zeolite catalyst ZSM-5 converts alcohols directly to gasoline (petrol) by dehydration which gives a mixture of hydrocarbons.
Question 33.
State the importance of colloids in day-to-day life.
Answer:
- Colloid chemistry is the chemistry of everyday life.
- A number of substances we use in our day-to-day life are colloids. For example, milk, butter, jelly, whipped cream, mayonnaise.
- Knowledge of colloid chemistry is essential for understanding about many useful materials like cement, bricks, pottery, porcelain, glass, enamels, oils, lacquers, rubber, celluloid and other plastics, leather, paper, textiles, filaments, crayons, inks, road construction material, etc.
- In many daily processes like cooking, washing, dyeing, painting, ore floatation, water purification, sewage disposal, smoke prevention, photography, pharmacy, use of colloids is important.
![]()
Question 34.
What are colloids? Explain.
Answer:
i. Colloids are heterogeneous mixtures.
ii. The component of colloid present in the largest proportion is called dispersion medium and the other components are called dispersed phase.
iii. The particles of the dispersed phase are larger than the size of a molecule and smaller than the particles which we can see with naked eye.
e.g.
- Observe the formation of solution of salt and water. Salt dissolves completely in water and forms homogeneous system.
- On the other hand, ground coffee or tea leaves with milk form suspension.
- Between the two extremes of solution and suspension exists a large group of systems called colloidal dispersions or simply colloids.
Question 35.
State the differences between colloids and solutions.
Answer:
Colloids:
- Colloids contain particles of dispersed phase with diameters in the range of 2 to 500 nm.
- They are translucent to light.
- e.g. Milk, fog, etc.
Solutions:
- Solutions contain solute particles with diameters in the range of 0.1 to 2 nm.
- They are transparent or may be coloured.
- e.g. NaCl solution
Question 36.
Explain: Natural phenomena of colloids observed in daily life.
Answer:
Following are some examples of colloids observed in daily life.
i. Blue colour of the sky: The sky appears blue to us because minute dust particles along with minute water droplets dispersed in air scatter blue light which reaches our eyes.
ii. Blood: It is a colloidal dispersion of plasma proteins and antibodies in water arid at the same time blood is also a suspension of blood cells and platelets in water.
iii. Soils: Fertile soils are colloidal in nature where humus acts as a protective colloid. Soil adsorbs moisture and nourishing materials due to its colloidal nature.
iv. Fog, mist and rain:
- Mist is caused by small droplets of water dispersed in air.
- Fog is formed whenever there is temperature difference between ground and air.
- A large portion of air containing dust particles gets cooled below its dew point, the moisture from the air condenses on the surface of these particles which form fine droplets, which are colloidal particles and float in the air as fog or mist.
Question 37.
State different ways to classify colloids.
Answer:
Colloids can be classified in three different ways:
- Physical states of dispersed phase and dispersion medium
- Interaction or affinity of phases
- Molecular size
Question 38.
Name the types of colloids based on the physical states of dispersed phase and dispersion medium. Give two examples of each.
Answer:
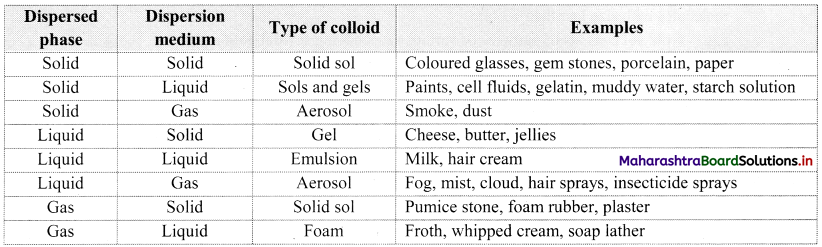
There are eight types of colloids based on the physical states of dispersed phase and dispersion medium as given below.
| Sr. No. | Type of Colloids | Examples |
| i. | Solid sol (solid dispersed in solid) | Coloured glasses, gemstones |
| ii. | Sols and gels (solid in liquid) | Gelatin, muddy water |
| iii. | Aerosol (solid in gas) | Smoke, dust |
| iv. | Gel (liquid in solid) | Cheese, jellies |
| v. | Emulsion (liquid in liquid) | Milk, hair cream |
| vi. | Aerosol (liquid in gas) | Fog, mist |
| vii. | Solid sol (gas in solid) | Foam rubber, plaster |
| viii. | Foam (gas in liquid) | Froth, soap lather |
![]()
Question 39.
Complete the following chart.
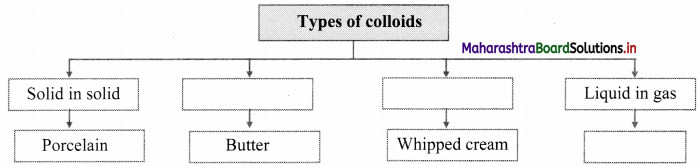
Answer:
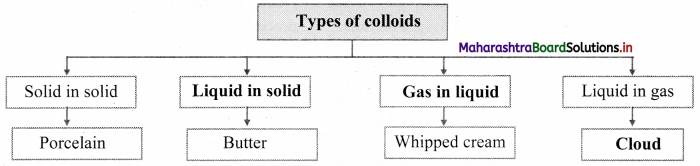
[Note: Students can write any one example of the given type of colloids.]
Note: Types of colloids based on the physical states of dispersed phase and dispersion medium.
Question 40.
Describe classification of colloids based on the interaction or affinity of phases.
Answer:
On the basis of interaction or affinity of phases, a colloidal solution is classified as lyophilic and lyophobic.
i. Lyophilic colloids:
- A colloidal solution in which the particles of dispersed phase have a great affinity for the dispersion medium are lyophilic colloids.
- If the lyophilic sol is evaporated, the dispersed phase separates. However, if it is remixed with the medium, the sol. can be formed again and hence, such sols are called reversible sols.
- They are stable and difficult to coagulate.
ii. Lyophobic colloids:
- Colloidal solution in which the particles of the dispersed phase have no affinity for the dispersion
medium are called lyophobic colloids. - The common examples are Ag, Au, hydroxides like Al(OH)3, Fe(OH)3, metal sulphides.
- Once precipitated or coagulated they have little tendency or no tendency to revert back to colloidal state.
[Note: Lyo means liquid and philic means loving whereas phobic means fearing and hence liquid hating. If water is the dispersion medium, the terms hydrophilic and hydrophobic are used.]
Question 41.
Give reason: Lyophilic sols are called reversible sols.
Answer:
- When lyophilic sol is evaporated, the dispersed phase separates.
- However, if the dispersed phase is remixed with the medium, the sol can be formed again.
Hence, lyophilic sols are called reversible sols.
Question 42.
How are colloids classified based on their molecular size?
Answer:
Colloids are classified into three types based on their molecular size as described below.
i. Multimolecular colloids:
- In multimolecular colloids, the individual particles consist of an aggregate of atoms or small molecules with size less than 103 pm.
e.g. Gold sol consists of particles of various sizes having several gold atoms. - Colloidal solution in which particles are held together with van der Waals force of attraction is called multimolecular colloid.
e.g. S8 sulphur molecules
ii. Macromolecular colloids: In this type of colloids, the molecules of the dispersed phase are sufficiently large in size (macro) to be of colloidal dimensions.
e.g. Starch, cellulose, proteins, polythene, nylon, plastics.
iii. Associated colloids or micelles:
- The substances behave as normal electrolytes at low concentration and associated in higher concentration forming a colloidal solution.
- The associated particles are called micelles, e.g. Soaps and detergents
![]()
Question 43.
How can be colloids prepared by chemical methods?
Answer:
i. Colloidal dispersions can be prepared by chemical reactions leading to formation of molecules by double decomposition, oxidation, reduction or hydrolysis.
ii. Molecules formed in these reactions are water-insoluble and thus, they aggregate leading to the formation of colloids.
e.g.
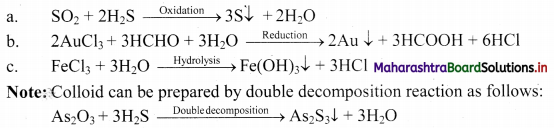
Question 44.
Describe the process involved in peptization?
Answer:
- During peptization a precipitate is converted into colloidal sol by shaking with dispersion medium in the presence of a small amount of an electrolyte. The electrolyte used is known as peptizing agent.
- During the process, the precipitate adsorbs one of the ions of the electrolyte on its surface and as a result, positive or negative charge is developed on the precipitate which finally breaks up into small particles of colloidal size.
[Note: This method is generally applied to convert a freshly prepared precipitate into a colloidal sol.]
Question 45.
Why is it necessary to purify colloidal solutions?
Answer:
- Colloidal solution generally contains excessive amount of electrolytes and some other soluble impurities.
- A small quantity of an electrolyte is necessary for the stability of colloidal solution, however, a large quantity of electrolyte may result in coagulation.
- It is also necessary to reduce soluble impurities.
Hence, it is necessary to purify colloidal solutions.
Question 46.
i. What is purification of colloidal solution?
ii. How can a colloidal solution be purified using the method of dialysis?
Answer:
i. The process used for reducing the amount of impurities to a requisite minimum is known as purification of colloidal solution.
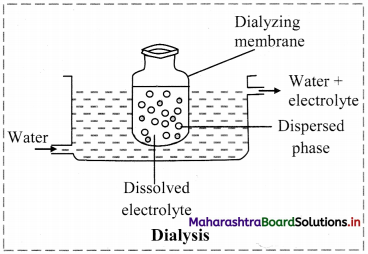
ii. a. Dialysis is a process of removing a dissolved substance from a colloidal solution by diffusion through a suitable membrane.
b. Purification of colloidal solution can be carried out using dialysis by the following method.
- The apparatus used is dialyser.
- A bag of suitable membrane containing the colloidal solution is suspended in a vessel through which fresh water is continuously flowing.
- The molecules and ions diffuse through membrane into the outer water and pure colloidal solution is left behind.
Question 47.
What are the general properties exhibited by colloidal dispersions?
Answer:
General properties exhibited by colloidal dispersions:
- Colloidal system is heterogeneous and consists of two phases, dispersed phase and dispersion medium.
- The dispersed phase particles pass slowly through parchment paper or animal membrane, but readily pass through ordinary filter paper.
- Colloidal particles are usually not detectable by powerful microscope.
![]()
Question 48.
Discuss the factors that influence the colour of colloidal solutions.
Answer:
- Colour of colloidal solution depends on the wavelength of light scattered by dispersed particles.
- The colour of colloidal dispersion also changes with the manner in which the observer receives the light.
e.g. Mixture of a few drops of milk and large amount of water appears blue when viewed by the scattered light and red when viewed by transmitted light. - It also depends on size of colloidal particles.
e.g. Finest gold sol is red in colour whereas with increase in size it appears purple.
Question 49.
Give three examples each:
i. Positively charged sols
ii. Negatively charged sols
Answer:
i. Positively charged sols: Al2O3. xH2O, haemoglobin, TiO2 sol
ii. Negatively charged sols: Au sols, Congo red sol, clay
Note: Some common sols with the nature of charge on the particles are listed in the table below.
| Positively charged sols | Negatively charged sols |
| Hydrated metallic oxides: Al2O3.xH2O, CrO3.xH2O, Fe2O3.xH2O. | Metals: Cu, Ag. Au sols Metallic sulphides: As2S3, Sb2S3, CdS |
| Basic dye stuff, methylene blue sols | Acid dye stuff, eosin, Congo red sol |
| Haemoglobin (blood) | Sols of starch, gum |
| Oxides: TiO2 sol | Gelatin, clay, gum sols |
Question 50.
Explain the term electroosmosis.
Answer:
- Movement of dispersed particles can be prevented by suitable means such as use of membrane.
- On doing so, it is observed that the dispersion medium begins to move in an electric field. This is known as electroosmosis.
Question 51.
What is coagulation?
Answer:
The precipitation of colloids by removal of charge associated with colloidal particles is called coagulation.
Question 52.
How can we bring about precipitation of lyophobic colloids?
Answer:
- The charge on the colloidal particles is due to the preferential adsorption of ions on their surface.
- Hence, lyophobic colloids can be precipitated out by removing the charge on the colloidal particles (dispersed phase).
![]()
Question 53.
Discuss various methods that are used to bring about coagulation of lyophobic sols.
Answer:
Coagulation of the lyophobic sols can be carried out in the following ways.
- By electrophoresis: The colloidal particles move towards oppositely charged electrodes, get discharged and precipitate.
- By mixing two oppositely charged sols: Oppositely charged sols when mixed in almost equal proportions neutralize their charges and get precipitated.
e. g. Mixing of hydrated ferric oxide (positive sol) and arsenious sulphide (negative sol) brings them in the precipitated forms. This type of coagulation is called mutual coagulation. - By boiling: When a sol is boiled, the adsorbed layer is disturbed as a result of increased collisions with molecules in the dispersion medium. This reduces charge on the particles and subsequently particles settle down as a precipitate.
- By persistent dialysis: On prolonged dialysis, traces of the electrolyte present in the sol are removed almost completely. The colloids then become unstable and finally precipitate.
- By addition of electrolytes: When excess of an electrolyte is added, the colloidal particles are precipitated.
Question 54.
Write Hardy-Schulze rule.
Answer:
Generally, greater the valency of the flocculating ion added, greater is its power to cause precipitation. This is known as Hardy-Schulze rule.
Question 55.
Differentiate between oil in water and water in oil emulsions.
Answer:
Oil in water:
- Oil is the dispersed phase and water is the dispersion medium.
- If water is added, it will be miscible with the emulsion.
- Addition of small amount of an electrolyte makes the emulsion conducting.
- Continuous phase is water.
- Basic metal sulphates, water soluble alkali metal soaps are used as emulsifiers.
Water in oil:
- Water is the dispersed phase and oil is the dispersion medium.
- If oil is added, it will be miscible with the emulsion.
- Addition of small amount of an electrolyte has no effect on conducting power.
- Continuous phase is oil.
- Water insoluble soaps such as those of Zn, Al, Fe, alkaline earth metals are used as emulsifiers.
Question 56.
What are the properties of emulsion?
Answer:
Properties of emulsion:
- Emulsion can be diluted with any amount of the dispersion medium. On the other hand, the dispersed liquid when mixed forms a separate layer.
- The droplets in emulsions are often negatively charged and can be precipitated by electrolytes.
- Emulsions show Brownian movement and Tyndall effect.
- The two liquids in emulsions can be separated by heating, freezing, centrifuging, etc.
Question 57.
Give applications of colloids.
Answer:
Applications of colloids:
i. Electrical precipitation of smoke:
- Smoke is a colloidal solution of solid particles of carbon, arsenic compound, dust, etc. in the air.
- When smoke is allowed to pass through chamber containing charged plates, smoke particles lose their charge and get precipitated. The particles then settle down on the floor of the chamber.
- The precipitator used is called Cottrell precipitator.
ii. Purification of drinking water:
- Water obtained from natural sources contains colloidal impurities.
- By addition of alum to such water, colloidal impurities get coagulated and settle down. This makes water potable.
iii. Medicines:
- Usually medicines are colloidal in nature.
- Colloidal medicines are more effective owing to large surface area to volume ratio of a colloidal particle and easy assimilation.
e.g. Argyrol is a silver sol used as an eye lotion. Milk of magnesia, an emulsion is used in stomach disorders.
iv. Rubber industry: Rubber is obtained by coagulation of latex.
v. Cleansing action of soaps and detergents.
vi. Photographic plates, films, and industrial products like paints, inks, synthetic plastics, rubber, graphite lubricants, cement, etc. are colloids.
![]()
Question 58.
Match column A with column B.
| Column A | Column B | ||
| i. | Tyndall effect | i. | Kinetic property |
| ii. | Electrophoresis | ii. | Argyrol |
| iii. | Silver sol | iii. | Optical property |
| iv. | Brownian motion | iv. | Coagulation |
Answer:
i – c,
ii – d,
iii – b,
iv – a
Question 59.
In drinking water treatment, often alum is added for the complete removal of suspended impurities. On complete dissolution, alum produces positive charge which neutralizes the charge on the suspended particles and thus, impurities are easily removed.
i. Name and define the process involved due to which charge on particles get neutralized.
ii. What is the role of alum in the above mentioned process?
Answer:
i. a. Charge on particles get neutralized due to coagulation.
b. The precipitation of colloids by removal of charge associated with colloidal particles is called coagulation.
ii. Alum acts as a reagent that helps in coagulation of the suspended particles by the removal of the charge associated with these particles.
Multiple Choice Questions
1. Which of the following is responsible for adsorption phenomenon?
(A) Hydrogen bonding
(R) Dipole-dipole forces
(C) Ion-dipole forces
(D) Dispersion forces
Answer:
(D) Dispersion forces
2. A substance which adsorbs another substance on its surface is called ……………..
(A) adsorbate
(B) absorbate
(C) adsorbent
(D) absorbent
Answer:
(C) adsorbent
3. During adsorption, the molecules of the substance which gets adsorbed are termed as
(A) adsorbent
(B) adsorbate
(C) absorbent
(D) absorbate
Answer:
(B) adsorbate
![]()
4. in adsorption of acetic acid on charcoal, acetic acid is ……………
(A) adsorhate
(B) adsorbent
(C) absorbent
(D) absorbate
Answer:
(A) adsorhate
5. The process of removal of an adsorbed substance from the surface is known as
(A) sorption
(B) oxidation
(C) reduction
(D) desorption
Answer:
(D) desorption
6. ………….. is the process in which adsorbate molecules are held on the surface of the adsorbent by weak van der Waals forces.
(A) Chemisorption
(B) Absorption
(C) Physisorption
(D) Biosorption
Answer:
(C) Physisorption
7. Which of the following is an example of physical adsorption?
(A) Adsorption of acetic acid in solution by charcoal
(B) Adsorption of O2 on tungsten
(C) Adsorption of N2 on Fe
(D) Adsorption of H2 on Ni
Answer:
(A) Adsorption of acetic acid in solution by charcoal
8. Chemisorption is a slow process because …………….
(A) it forms multimolecular layer
(B) it is reversible
(C) it takes place at normal temperature
(D) it requires high activation energy
Answer:
(D) it requires high activation energy
9. The number of layer(s) formed on adsorbent in chemical adsorption is …………….
(A) one
(B) two
(C) three
(D) many
Answer:
(A) one
![]()
10. Which of the following statements is CORRECT regarding chemical adsorption?
(A) It is highly specific in nature.
(B) It is relatively strong.
(C) It involves the formation of monolayer of adsorbed particles.
(D) All of these.
Answer:
(D) All of these.
11. Which of the following is adsorbed to maximum extent on charcoal?
(A) H2
(B) N2
(C) Cl2
(D) O2
Answer:
(C) Cl2
12. The relation between the amount of substance adsorbed by an adsorbent and the equilibrium pressure or …………. at any constant temperature is called adsorption isotherm.
(A) surface area
(B) volume
(C) circumference
(D) concentration
Answer:
(D) concentration
13. For equilibrium pressure (P), the mass of gas adsorbed (x) and mass of adsorbent (m) may be expressed as Freundlich adsorption isotherm as ……………
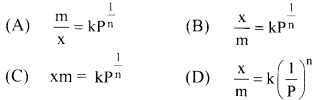
Answer:
(B) \(\frac{\mathrm{x}}{\mathrm{m}}=\mathrm{kP}^{\frac{1}{\mathrm{n}}}\)
14. When log x/m is plotted against log P, the intercept obtained …………..
(A) on Y axis is equal to log K
(B) on Y axis is equal to K
(C) on X axis is equal to log K
(D) on X axis is equal to K
Answer:
(A) on Y axis is equal to log K
15. The adsorption isotherm tends to saturate at ………….. pressure.
(A) low
(B) moderate
(C) all of these
(D) high
Answer:
(D) high
![]()
16. In Haber process for manufacture of NH3, the catalyst used is ……………
(A) iron
(B) copper
(C) vanadium pentoxide
(D) nickel
Answer:
(A) iron
17. A substance that decreases the rate of a chemical reaction is called ……………
(A) inhibitor
(B) prohibitor
(C) promoter
(D) reactor
Answer:
(A) inhibitor
18. Whether a given mixture forms a true solution or a colloidal dispersion depends on the …………….
(A) charge of solute particles
(B) size of solvent particles
(C) size of solute particles
(D) charge of solvent particles
Answer:
(C) size of solute particles
19. An aerosol is a dispersion of a ……………
(A) gas in a solid
(B) liquid in a gas
(C) solid in a gas
(D) both (B) and (C)
Answer:
(D) both (B) and (C)
20. The dispersed phase in Pumice stone is ……………
(A) solid
(B) liquid
(C) gas
(D) none of these
Answer:
(C) gas
21. Colloidal solution in which the dispersed phase has little affinity for the dispersion medium is called ………………
(A) lyophobic colloids
(B) lyophilic colloids
(C) hydrophilic colloids
(D) emulsions
Answer:
(A) lyophobic colloids
![]()
22. Which of the following is NOT an example of macromolecular colloid?
(A) Starch
(B) Proteins
(C) S8 molecules
(D) Nylon
Answer:
(C) S8 molecules
23. Tyndall effect is useful ……………….
(A) to identify colloidal dispersions
(B) to count number of particles in colloidal dispersion.
(C) to determine the size of the colloidal particles
(D) all of these
Answer:
(D) all of these
24. Brownian movement is a ……………… type of property of the colloidal sol.
(A) electrical
(B) optical
(C) kinetic
(D) colligative
Answer:
(C) kinetic
25. The migration of colloidal particles under the influence of an electric field is called …………….
(A) catalysis
(B) Brownian movement
(C) electrophoresis
(D) Tyndall effect
Answer:
(C) electrophoresis
26. The capacity of an ion to coagulate a colloidal solution depends on ……………….
(A) its shape
(B) its valency
(C) the sign of charge
(D) both (B) and (C)
Answer:
(D) both (B) and (C)
![]()
27. ……………… is an example of water in oil type of emulsion.
(A) Milk
(B) Cod liver oil
(C) Vanishing cream
(D) Paint
Answer:
(B) Cod liver oil
28. Which of the following has highest precipitation power to precipitate negative sol?
(A) Al3+
(B) Mg2+
(C) Na+
(D) K+
Answer:
(A) Al3+